1 Global Ketamine API Market Insight Analysis
The global ketamine API market is valued at USD 65.03 million in 2024, with a CAGR of 26.12% from 2024 to 2033.
Ketamine API refers to the raw material drug used in the production of Ketamine preparations, which is the active ingredient in the preparations.
Figure Global Ketamine API Market Size (M USD) and CAGR (2024-2033)

2 Ketamine API Market Growth Drivers and Restraints
Drugs are widely used in many fields: Ketamine is widely used as an anesthetic in surgical operations. It can be used for anesthesia for tissue resection, fracture reduction and fixation, surgical debridement and other operations. It can also be used as an induction agent or auxiliary drug for other general anesthesia, which makes the demand for ketamine APIs in surgical operations stable. At the same time, ketamine is also used in the treatment of chronic pain and sedation of ICU patients, further broadening its demand areas.
In recent years, ketamine has emerged in the field of depression treatment. Low-dose injection can quickly improve depression symptoms, and the effect lasts for several days to two weeks. There are many ketamine treatment clinics in the United States, and relevant studies have shown that it is expected to become a legal depression treatment drug, which has greatly promoted the market demand for ketamine APIs and promoted market growth.
Increase in depression patients and treatment needs: Depression has become a serious global public health problem. According to data from the World Health Organization, about 3.8% of the population suffers from it. With the increase of life pressure and the intensification of social competition, the number of patients with depression continues to rise. Existing mainstream antidepressants have defects, while ketamine not only has a fast onset and long-lasting effect, but also has fewer mental side effects. As the research deepens, its enantiomers S-ketamine and R-ketamine enter the field of antidepressant treatment research, which makes ketamine more advantageous in the antidepressant drug market, thereby driving the development of the ketamine API market.
The development of generic drugs drives demand: In emerging markets, such as India, the government strongly supports the development of generic drugs, and the scale of the generic drug market continues to expand. The increase in the production of ketamine generic drugs has increased the demand for ketamine APIs and promoted market growth. From a global perspective, generic drugs play an important role in reducing medical costs. Their development has enabled more patients to afford ketamine-related drugs, indirectly increasing the demand for APIs.
Strict industry supervision: Ketamine has a certain psychological dependence and has been abused in some entertainment venues. To prevent it from flowing into illegal channels, governments of various countries have implemented strict supervision on ketamine APIs. In China, ketamine APIs are classified as Class II psychotropic drugs, which are produced by pharmaceutical companies designated by the State Food and Drug Administration. Other units and individuals are prohibited from producing them, and they can only be sold directly by manufacturers to preparation manufacturers. Distributors are not allowed to operate without authorization. The US FDA also strictly regulates the industry. This restricts new companies from entering the market, increases corporate compliance costs, and to some extent affects the market’s expansion speed.
Raw material price fluctuations and supply risks: The raw materials required for the production of ketamine APIs, such as hydroxyl imine, o-chlorophenyl cyclopentyl ketone and related catalysts, are subject to fluctuation risks in supply and price. Due to the characteristics of the industry, API manufacturers rely on major suppliers to purchase raw materials.
If the cooperative relationship with suppliers deteriorates, or if there are problems with the supplier’s operating and financial conditions, the company may not be able to purchase raw materials in a timely and sufficient manner, affecting production plans. Rising raw material prices will increase production costs and compress product profit margins. Companies may be forced to adjust their production and operation plans, which has become an obstacle to market growth.
3 Technological Innovations in the Ketamine API Market
New drug development: Ketamine is a racemic mixture of S-ketamine and R-ketamine, of which S-ketamine has a higher affinity for NMDA receptors and may have a stronger antidepressant effect. In 2015, Hassamal et al. showed that S-ketamine has long-term efficacy in patients with treatment-resistant depression and has no obvious side effects. Esketamine, as a form of S-ketamine, has been validated for efficacy and safety by multiple randomized controlled clinical trials and was approved by the US FDA in 2019 for treatment-resistant depression in adults. This is the first new antidepressant approved by the FDA in the past decade. This has prompted market companies to increase their research on S-ketamine APIs and promote product innovation.
Production process optimization: In the production process of ketamine, existing catalysts have problems such as poor applicability, low conversion number, poor stability and difficulty in recycling, and the catalytic efficiency and selectivity are often further reduced after the catalyst is immobilized. Therefore, developing new and efficient chiral catalysts and improving mass transfer efficiency and chiral selectivity in chiral catalytic reactions have become the trend of industry technology development. This will not only help improve the production efficiency and quality of ketamine APIs, but also reduce production costs and enhance the competitiveness of enterprises in the market.
Enhance market competitiveness: On January 1, 2021, Yonsung Group successfully acquired Arevipharma. Arevipharma has a long history of nearly 150 years and is an internationally renowned production base. The acquisition enables both parties to achieve synergies in R&D and industrial networks. The Radebeul plant has become the core hub of both parties’ API business in Europe and the United States. Through backward integration of production, product quality, customer service and supply chain stability are ensured, which enhances the competitiveness of enterprises in the market.
Promote industry integration: On December 17, 2021, SK Capital Partners, a private equity investment company focusing on specialty materials, chemicals and pharmaceuticals, acquired a majority stake in Seqens, which subsequently merged with Wavelength Pharmaceuticals under SK Capital. The combined Seqens has enhanced its capabilities in the pharmaceutical services and specialty ingredients markets, with a richer product portfolio covering 200 pharmaceutical ingredients, 500 intermediates and key specialty ingredients and chemicals. This merger has promoted industry consolidation, changed the market competition landscape, promoted the optimal allocation of resources, further consolidated the dominant position of large enterprises in the market, and had an important impact on the development direction of the industry.
4 Global Ketamine API Market Size by Type
Ketamine Hydrochloride is a widely used form of Ketamine API, primarily utilized for its analgesic and anesthetic properties. In 2024, the revenue generated by Ketamine Hydrochloride is forecasted to be 21.37 million USD. This represents a significant portion of the total market revenue, accounting for approximately 32.86% of the global Ketamine API market. The growth of Ketamine Hydrochloride is driven by its extensive use in surgical procedures, emergency medicine, and chronic pain management. Its stable hemodynamic profile and rapid onset of action make it a preferred choice for many medical applications.
Esketamine Hydrochloride, on the other hand, is gaining prominence due to its unique therapeutic benefits, particularly in the treatment of depression and other mood disorders. In 2024, Esketamine Hydrochloride is expected to generate a revenue of 43.66 million USD, which is significantly higher than that of Ketamine Hydrochloride. This type accounts for 67.14% of the total market revenue. The rapid growth of Esketamine Hydrochloride can be attributed to its approval by regulatory bodies such as the FDA for the treatment of treatment-resistant depression. Additionally, its application in various forms, including nasal sprays and injections, has expanded its market reach and acceptance.
Table Global Ketamine API Market Size and Share by Type in 2024
Type | Market Size (M USD) 2024 | Market Share 2024 |
|---|---|---|
Ketamine Hydrochloride | 21.37 | 32.86% |
Esketamine Hydrochloride | 43.66 | 67.14% |
5 Global Ketamine API Market Size by Application
Analgesic & Anesthetic is a major application area for Ketamine API, leveraging its properties as a potent anesthetic and pain management drug. In 2024, the revenue generated from this application is forecasted to be 18.09 million USD, accounting for approximately 27.82% of the global Ketamine API market revenue. The use of Ketamine API in this segment is driven by its effectiveness in providing rapid and reliable anesthesia, especially in surgical procedures and emergency medical situations. Its ability to maintain stable hemodynamics and provide analgesia makes it a preferred choice in pre-hospital care, operating rooms, and intensive care units.
Anti-depressant is another significant application area for Ketamine API, particularly with the growing recognition of its efficacy in treating depression and other mood disorders. In 2024, the revenue from the anti-depressant application is expected to reach 46.94 million USD, representing 72.18% of the total market revenue. The rapid growth in this segment is attributed to the increasing prevalence of depression and the need for effective treatment options. The FDA’s approval of Esketamine (an enantiomer of Ketamine) for treatment-resistant depression has further bolstered the market for Ketamine API in this application. Its quick onset of action and long-lasting effects make it an attractive option for patients and healthcare providers.
Table Global Ketamine API Market Size and Share by Application in 2024
Application | Market Size (M USD) 2024 | Market Share 2024 |
|---|---|---|
Analgesic & Anesthetic | 18.09 | 27.82% |
Anti-depressant | 46.94 | 72.18% |
6 Global Ketamine API Market Size by Region
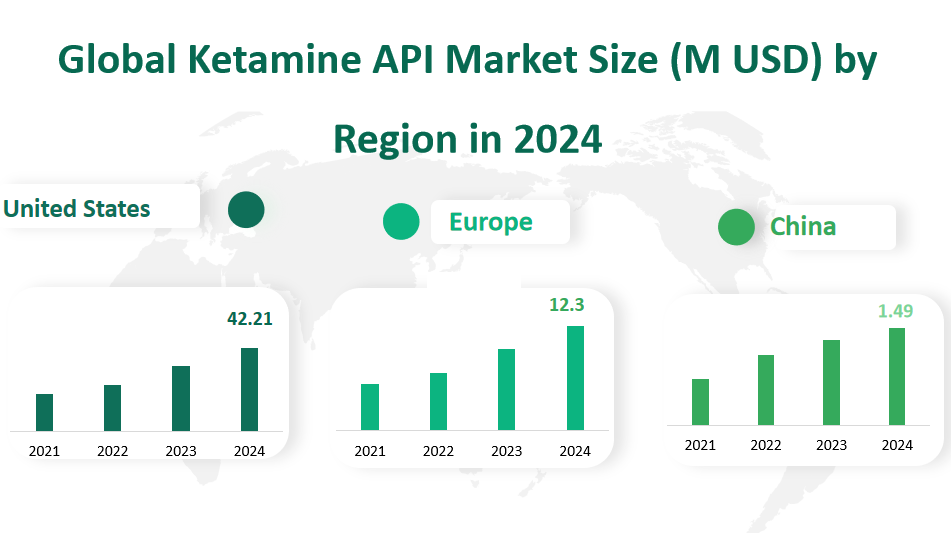
In 2024, the United States is projected to dominate the Ketamine API market with a substantial revenue of 42.21 million USD. This represents a significant growth from the previous years, indicating a strong demand for Ketamine API in the region. The U.S. market’s robust performance can be attributed to several factors, including advanced healthcare infrastructure, high healthcare spending, and a greater inclination towards innovative pharmaceutical solutions. The country’s significant investment in research and development (R&D) further fuels the market, as does the presence of key pharmaceutical companies that are actively involved in the production and distribution of Ketamine API.
Europe is another crucial market for Ketamine API, with a projected revenue of 12.3 million USD in 2024. The growth in the European market is driven by a combination of factors, including a well-established healthcare system, increasing awareness of mental health issues, and the availability of novel treatment options. The European region’s regulatory environment, which supports the approval and adoption of new drugs, also contributes to the market’s expansion. Additionally, the presence of a large patient population that requires effective pain management and antidepressant treatments further bolsters the demand for Ketamine API.
China is an emerging market for Ketamine API, with a projected revenue of 1.49 million USD in 2024. The growth in China’s market is indicative of the broader trends in healthcare spending and the government’s focus on improving access to quality healthcare services. The increasing prevalence of chronic pain and mental health disorders, coupled with the rising disposable income and urbanization, are driving the demand for Ketamine API. China’s strategic initiatives to bolster its pharmaceutical industry, including investments in domestic production capabilities and partnerships with international pharmaceutical companies, are also contributing to the market’s development.
Figure Global Ketamine API Market Size (M USD) by Region in 2024
7 Global Ketamine API Market Analysis by Major Players
Supriya Lifescience
Company Profile: Supriya Lifescience, established in 1987, is a prominent player in the API manufacturing industry. The company operates from its plants in India and has a global sales reach, catering to clients across the world.
Business Overview: Supriya Lifescience focuses on delivering quality products by adhering to industry standards and continuously improving their processes. The company has a solid position in the API manufacturing field, offering products in various therapeutic areas such as antihistamines, antiallergics, vitamins, anesthetics, anti-asthmas, and more. Their commitment to quality and innovation has helped them establish a strong presence in the market.
Product Offered: Supriya Lifescience offers Ketamine Hydrochloride USP/EP/BP/IP, which is used for its analgesic and anesthetic activities. The product is known for its ability to non-competitively block N-methyl-D-aspartate (NMDA) receptors, interacting with opioid mu receptors and sigma receptors, thereby reducing pain perception, inducing sedation, and producing dissociative anesthesia.
SEQENS
Company Profile: SEQENS, established in 2003, is a global leader in pharmaceutical solutions and specialty ingredients. The company operates 24 manufacturing plants and 10 R&D centers across Europe, North America, and Asia.
Business Overview: SEQENS offers a broad portfolio of active ingredients, pharmaceutical intermediates, and specialty ingredients. The company is known for its ability to innovate, develop, and implement the best available technologies. During the pandemic, SEQENS leveraged its expertise in the synthesis of active ingredients, intermediates, and precursors, contributing significantly to the pharmaceutical industry.
Product Offered: SEQENS provides Ketamine HCl with capabilities that include a total reactor capacity of 92 m³, reaction vessels ranging from 1 to 6 x 300 L, and a state-of-the-art cGMP facility. They offer flagship technologies such as hydrogenation, reductive amination, organometallic reactions, and more.
Maithri Drugs
Company Profile: Maithri Drugs, established in 2002, is one of the fastest-growing pharmaceutical companies in India. The company operates plants in India and serves clients mainly in North America, Asia, and Europe.
Business Overview: Maithri Drugs focuses on active pharmaceutical ingredients (APIs) and is recognized for its R&D excellence and aggressive growth strategy. The company’s facilities are audited and approved by the US FDA and are certified to DCGI, WHO GMP, and ISO 9001:2015 standards.
Product Offered: Maithri Drugs offers Esketamine Hydrochloride, categorized under antiepileptics, and Ketamine, categorized under anesthetics. These products are used for their therapeutic benefits in treating epilepsy and providing anesthesia.

