1 Global Rabies Monoclonal Antibody Market Insight Analysis
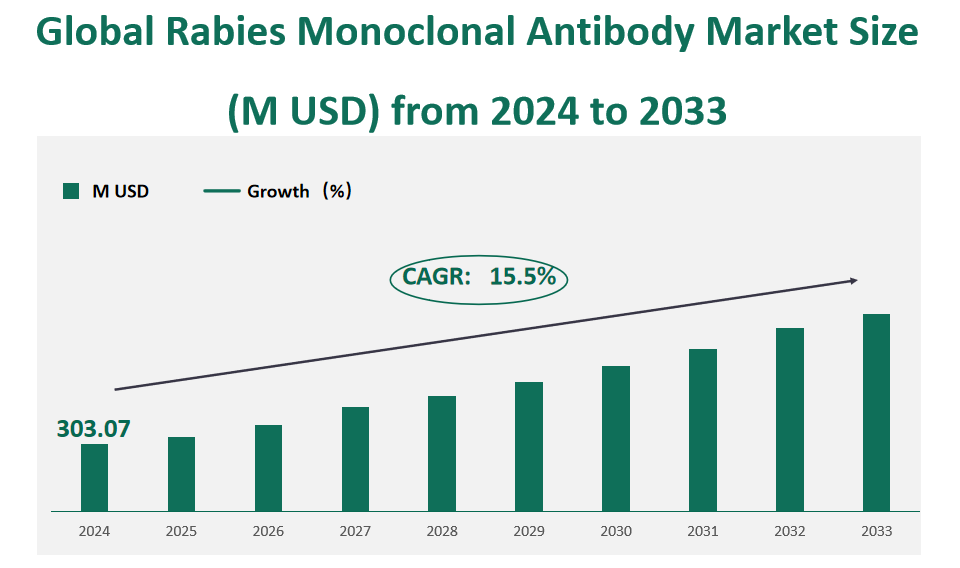
The global rabies monoclonal antibody market size was valued at USD 303.07 million in 2024, with a CAGR of 15.5% from 2024 to 2033.
Rabies virus (RABV), a single-stranded RNA virus of the genus Lyssavirus, family Rhabdoviridae, invades the central nervous system, causing acute and zoonotic natural foci disease worldwide. Rabies Monoclonal Antibody contains only antibodies against one or two epitopes, becoming one of the best options for post-exposure prophylaxis of rabies. Rabies Monoclonal Antibody has the advantages of high neutralizing activity, strong specificity to the corresponding target, easy standardized production, etc., and has broad application prospects.
Figure Global Rabies Monoclonal Antibody Market Size (M USD) and CAGR (2024-2033)
2 Rabies Monoclonal Antibody Market Growth Drivers and Restraints
Large-scale production advantages: Compared with traditional human rabies immunoglobulin (HRIG), rabies monoclonal antibodies have significant production advantages. HRIG relies on the extraction of blood antibodies from plasma donors after vaccination when the blood antibody reaches a certain level. The supply of plasma raw materials is limited, resulting in limited production and high prices. Rabies monoclonal antibodies are produced with the help of large-scale molecular technology, without relying on animal plasma, and the output is not limited. With the increase in the number of pet owners, the market demand for rabies prevention continues to expand, and HRIG cannot meet the market demand, providing a broad market space for rabies monoclonal antibodies.
No risk of blood-borne infection: As a blood product, HRIG has the potential risk of spreading blood-borne diseases such as AIDS, hepatitis B, and hepatitis C during the production process. In contrast, rabies monoclonal antibodies use genetic engineering recombination technology to achieve industrial scale and standardized production, without the participation of living organisms and blood, avoiding the risk of blood contamination. With the improvement of people’s awareness of the hazards of rabies and the increase in the number of pet owners, the demand for safe and effective rabies prevention products has increased, which makes rabies monoclonal antibodies stand out in the market competition with their advantage of no risk of blood-borne infection.
Policy support and institutional promotion: The World Health Organization (WHO) has been committed to the research and development of anti-rabies monoclonal antibodies since the 1990s, aiming to find products that replace rabies immunoglobulins (RIGs) for post-exposure prophylaxis (PEP). The Drug Evaluation Center of the China Food and Drug Administration has issued relevant documents to standardize and guide the clinical trials of anti-rabies virus monoclonal antibody drugs, which has promoted the development of the domestic rabies monoclonal antibody market. These policy supports and institutional promotions have provided strong external support for market growth.
Difficulties in clinical trials: Clinical trials of rabies monoclonal antibodies face many challenges. Although the animal bite rate is high in clinical practice, the actual incidence of rabies is low, and once the disease occurs, the mortality rate is almost 100%, and there is a lack of clear diagnostic methods during the incubation period.
This means that most of the exposed people included in the clinical trial may not be infected with the rabies virus, affecting the accuracy of the test results. In addition, rabies monoclonal antibodies are usually used in combination with vaccines, and how to evaluate their efficacy alone has become a problem. At the same time, the reasonable setting of follow-up periods, the selection of controls and subjects, etc. also need to be solved. These difficulties have hindered the development and listing of products.
Long R&D cycle: The rabies monoclonal antibody industry is a high-tech, high-risk, and high-investment field. From new drug development to market launch, it is necessary to go through multiple stages such as compound development, preclinical testing, IND application, clinical phase I, II, and III trials, registration and listing. Each link requires a large amount of capital, technology, and human resources, and the comprehensive quality requirements of R&D personnel are also very high. At present, the number of related products approved for marketing in India and China is limited, and most participants are still in the R&D stage. The long R&D cycle restricts the rapid development of the market.
3 Technological Innovations in the Rabies Monoclonal Antibody Market
Production technology innovation: At present, rabies monoclonal antibodies are mainly prepared through hybridoma technology, antibody library technology and B cell immortalization technology. Taking hybridoma technology as an example, its preparation process includes preparing hybridoma cells, establishing cell lines, sub-culturing, and finally preparing, identifying and verifying antibodies.
The application of these technologies enables the production of rabies monoclonal antibodies, but there is still room for improvement. In the future, technological innovation will focus on optimizing production processes, improving antibody yield and quality, and reducing production costs. For example, continuously improving cell culture conditions and developing more efficient cell screening methods to obtain monoclonal antibodies with better performance.
Product portfolio optimization: In order to improve the preventive effect of rabies monoclonal antibodies, the industry focuses on strengthening product research. On the one hand, cell culture technology is used to prepare safer, more active and more stable antibody drugs on a large scale; on the other hand, multiple monoclonal antibodies targeting different antigenic sites of the virus are made into combined preparations to deal with different virus strains or genotypes of viruses to ensure the effectiveness of the product against various rabies viruses. This is an important direction of current technological innovation in the industry.
4 Global Rabies Monoclonal Antibody Market Size by Type
Category II Exposure refers to minor wounds such as superficial scratches or abrasions without bleeding. This segment is characterized by a lower severity of rabies exposure but still requires prophylactic treatment to prevent the disease. In 2024, the market value for Category II Exposure is projected to reach 31.09 million USD. This segment has been growing steadily, driven by the increasing awareness of rabies prevention and the need for effective post-exposure prophylaxis even in less severe cases. The market share of Category II Exposure is expected to remain relatively stable, accounting for approximately 10.26% of the total market value in 2024.
Category III Exposure includes more severe incidents such as transdermal bites or scratches, contamination of mucous membranes, or exposure to bats. This segment represents the majority of the market due to the higher risk and severity of rabies transmission. In 2024, the market value for Category III Exposure is projected to reach 271.98 million USD, accounting for 89.74% of the total market value. The significant market share of Category III Exposure is driven by the critical need for effective and immediate treatment in severe cases, which often requires higher doses of monoclonal antibodies.
Table Global Rabies Monoclonal Antibody Market Size by Type in 2024
Type | Market Size (M USD) 2024 |
|---|---|
Category II Exposure | 31.09 |
Category III Exposure | 271.98 |
5 Global Rabies Monoclonal Antibody Market Size by Application
The adult segment represents a substantial portion of the rabies monoclonal antibody market. In 2024, the market value for the adult application is projected to reach 213.75 million USD, accounting for approximately 70.52% of the total market value. This segment is driven by the higher incidence of rabies exposure among adults, particularly in regions with high rates of animal bites and limited access to preventive measures. Adults are more likely to engage in activities that increase their risk of exposure, such as working with animals or traveling to areas with high rabies prevalence.
The growth in the adult segment is also supported by increasing awareness of rabies prevention and the availability of effective monoclonal antibody treatments. The market share of the adult segment is expected to remain stable or slightly increase due to ongoing efforts to improve healthcare infrastructure and access to rabies prophylaxis in developing countries.
The children segment is another critical application area for rabies monoclonal antibodies. In 2024, the market value for the children application is projected to reach 89.33 million USD, representing 29.48% of the total market value. Children are particularly vulnerable to rabies due to their close interactions with animals and their limited understanding of the risks associated with animal bites. The need for effective and safe rabies prophylaxis for children is a significant driver of this segment.
The market for children’s applications is growing steadily, supported by increased awareness among parents and healthcare providers about the importance of rabies prevention. Additionally, efforts to improve pediatric healthcare and vaccination programs in developing countries are contributing to the growth of this segment. The market share of the children segment is expected to remain stable, with potential for further growth as more regions implement comprehensive rabies prevention strategies.
Table Global Rabies Monoclonal Antibody Market Size by Application in 2024
Application | Market Size (M USD) 2024 |
|---|---|
Adult | 213.75 |
Children | 89.33 |
6 Global Rabies Monoclonal Antibody Market Size by Region
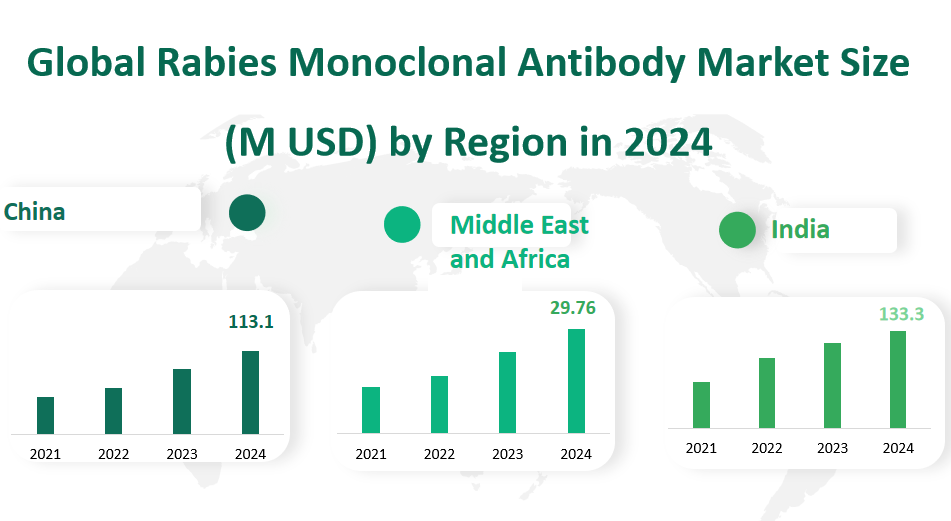
The market in China is projected to reach a revenue of USD 113.1 million in 2024. The chart indicates a steady growth trend since 2021, with an expected peak in 2024. This growth is primarily attributed to the Chinese government’s strong support for the biotechnology industry and the increasing demand for high-quality medical products domestically. Additionally, increased R&D investments in biotechnology have spurred advancements in monoclonal antibody production technologies by local companies. With the involvement of domestic enterprises such as North China Pharmaceutical Group’s New Drug Research and Development Co., Ltd., it is anticipated that China’s share in the global rabies monoclonal antibody market will continue to grow in the coming years.
The Middle East and Africa region is expected to see a significant increase, with the market size projected at USD 29.76 million for 2024. The upward trend in this region can be linked to the growing awareness of rabies prevention and the increasing demand for effective treatment options. The region’s market is also driven by the efforts of international health organizations and local governments to improve healthcare infrastructure and access to advanced medical treatments. Despite economic disparities and challenges in healthcare access in some areas, the Middle East and Africa are showing promising signs of growth in the rabies monoclonal antibody market.
India’s market is anticipated to boom in 2024, with an estimated revenue of USD 133.3 million. The chart shows a consistent upward trajectory since 2021, reflecting India’s expanding biotechnology sector and its role as a major producer of rabies monoclonal antibodies. Key players such as the Serum Institute of India contribute significantly to the market’s growth. India’s cost-effective production capabilities and extensive distribution networks position it as a key exporter of these products globally. The country’s focus on public health initiatives and the availability of advanced medical facilities are also driving the demand for rabies monoclonal antibodies.
Figure Global Rabies Monoclonal Antibody Market Size (M USD) by Region in 2024
7 Global Rabies Monoclonal Antibody Market Analysis by Major Players
Serum Institute of India
Company Profile: Established in 1966, Serum Institute of India is headquartered in Pune, India, and is recognized as one of the world’s largest manufacturers of vaccines.
Business Overview: The company specializes in the production of a wide range of vaccines using advanced genetic and cell-based technologies, including those for polio, diphtheria, tetanus, and COVID-19, in addition to rabies.
Product Offered: Rabishield, a rabies human monoclonal antibody indicated for post-exposure prophylaxis of rabies infection.
2023 Summary: Serum Institute of India reported sales of approximately 10,547 liters, with a revenue of USD 71.13 million and a gross margin of 29.52%.
Zydus
Company Profile: Founded in 1952, Zydus is an innovative, global pharmaceutical company based in India.
Business Overview: Zydus discovers, develops, manufactures, and markets a broad range of healthcare therapies, including biologic therapeutics and vaccines, with a focus on areas such as infectious diseases.
Product Offered: Twinrab 1500IU Injection, a combination medication containing docaravimab and miromavimab for post-exposure prophylaxis to prevent rabies infection.
2023 Summary: Zydus achieved sales of around 5,253 liters, generating a revenue of USD 59.09 million and maintaining a gross margin of 27.27%.
NCPC (North China Pharmaceutical Group Corporation)
Company Profile: NCPC, established in 1953, is based in Shijiazhuang City, Hebei Province, China, and operates with a strong presence in the pharmaceutical industry.
Business Overview: The company has a diverse portfolio of products in chemical pharmaceuticals, modern biotechnology drugs, and traditional Chinese medicine, with a focus on therapeutic areas such as anti-infection and immunity regulation.
Product Offered: Ormutivimab Injection, a recombinant human anti-rabies virus monoclonal antibody injection used for passive immunization of adults exposed to the rabies virus.
2023 Summary: NCPC recorded sales of about 1,227 liters, with a revenue of USD 82.84 million and a gross margin of 32.10%.

