1 Global Nitinol Medical Devices Market Outlook
The global Nitinol Medical Devices market is projected to exhibit substantial growth in the coming years, with a CAGR of 5.66% from 2024 to 2033, reaching a total market size of $19242 million USD in 2024. Nitinol, an alloy of nickel and titanium, is renowned for its superelasticity and shape memory properties. These characteristics make it an ideal material for various medical applications, particularly in minimally invasive procedures. Devices made from Nitinol, such as stents, guidewires, and orthopedic implants, offer superior flexibility, durability, and biocompatibility compared to traditional materials. The ability of Nitinol to return to its original shape after deformation, combined with its resistance to corrosion, makes it highly suitable for cardiovascular and orthopedic applications.
Figure Global Nitinol Medical Devices Market Size and Growth Rate (2024-2033)
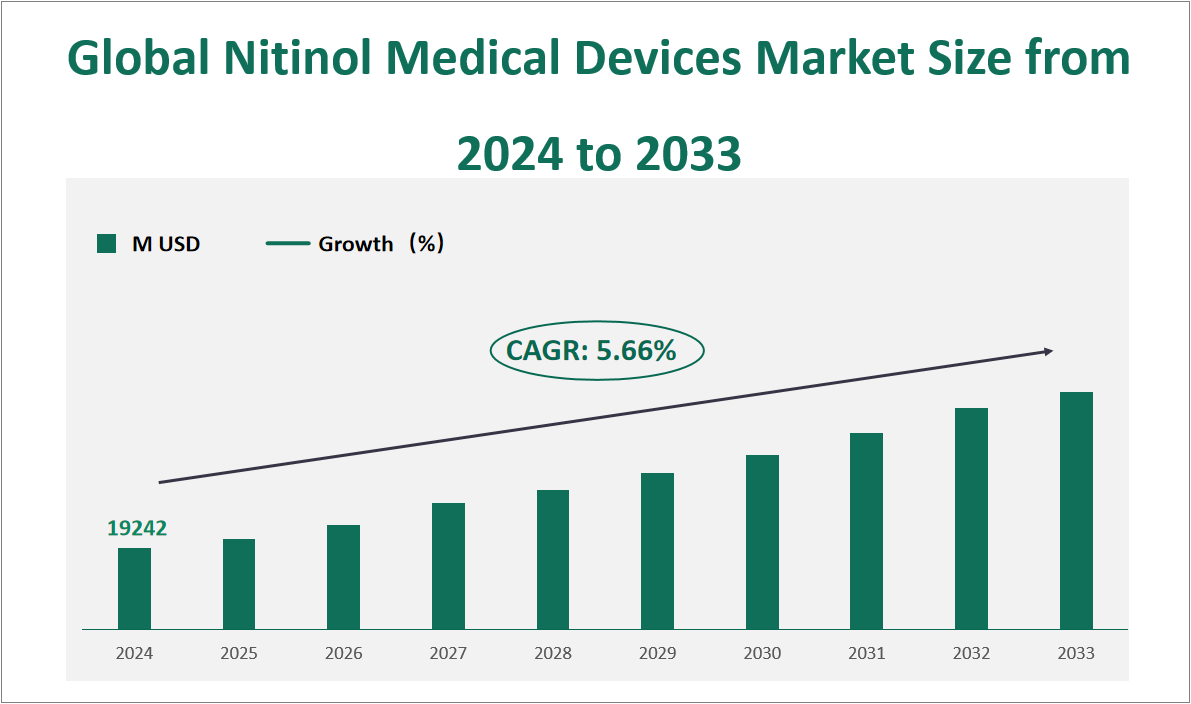
2 Nitinol Medical Devices Market Growth Drivers and Constraints
The growth of the global Nitinol Medical Devices market is influenced by several key factors. The primary drivers include the aging global population and the increasing prevalence of cardiovascular diseases. As the population ages, the demand for medical devices to treat conditions such as ischemic heart disease and stroke continues to rise. Nitinol-based devices, with their superior performance in minimally invasive procedures, are becoming increasingly important in addressing these health challenges.
Another significant driver is the growing popularity of minimally invasive surgeries. These procedures offer several advantages over traditional open surgeries, including reduced recovery time, lower risk of complications, and minimal scarring. Nitinol’s unique properties, such as its superelasticity and shape memory, make it an essential material for the development of devices used in these procedures. The material’s ability to navigate complex anatomical structures with minimal trauma further enhances its appeal in the medical device industry.
However, the market also faces several limiting factors. One of the primary challenges is the stringent regulatory environment governing medical devices. The U.S. Food and Drug Administration (FDA) and other regulatory bodies require extensive testing and validation of Nitinol devices to ensure their safety and efficacy. This includes evaluating the material’s biocompatibility, corrosion resistance, and mechanical properties. The complexity of the regulatory process can delay the introduction of new products and increase development costs.
Another limiting factor is the high barrier to entry in the medical device industry. Developing Nitinol-based devices requires significant investment in research and development, as well as specialized manufacturing capabilities. The production process for Nitinol devices is complex and requires precise control over material composition and processing conditions. These factors can deter new entrants and limit market competition.
3 Nitinol Medical Devices Market Innovations and M&A Activities
The Nitinol Medical Devices market is characterized by continuous technological innovation and strategic corporate activities. Companies are constantly investing in research and development to enhance the performance of existing devices and develop new applications for Nitinol. For instance, advancements in microelectromechanical systems (MEMS) and thin-film Nitinol technologies are opening up new possibilities for active medical implantable devices.
Corporate mergers and acquisitions are also a common trend in the industry. These strategic moves are aimed at expanding market share, acquiring new technologies, and strengthening product portfolios. For example, Boston Scientific’s acquisition of a majority stake in M.I.Tech Co., Ltd., a Korean manufacturer of medical devices, highlights the company’s commitment to expanding its presence in the minimally invasive device market. Similarly, Terumo’s commercial implant of the Thoraflex Hybrid Device in the United States demonstrates the company’s focus on innovative solutions for complex cardiovascular conditions.
In addition to mergers and acquisitions, companies are also forming strategic partnerships to drive innovation and market expansion. These collaborations often involve sharing resources, expertise, and intellectual property to accelerate the development and commercialization of new Nitinol-based devices. The competitive landscape is further shaped by the efforts of major players such as Abbott, Medtronic, and Edwards Lifesciences, who continue to invest in new product development and market penetration strategies.
In conclusion, the global Nitinol Medical Devices market is poised for steady growth driven by the increasing demand for minimally invasive procedures and the unique material properties of Nitinol. However, the market faces regulatory and technological challenges that require continuous innovation and strategic corporate activities to overcome. As the industry evolves, the focus on improving patient outcomes and expanding the application of Nitinol in medical devices will remain paramount.
4 Global Nitinol Medical Devices Market Analysis by Type
In 2024, the global Nitinol Medical Devices market is forecasted to reach a total revenue of $19,242 million USD. Among the different types of Nitinol Medical Devices, stents are expected to hold the largest market share, generating a revenue of $7,864 million USD, accounting for approximately 40.87% of the total market. Guidewires follow closely with a projected revenue of $6,086 million USD, representing 31.63% of the market. The remaining segment, categorized as “others,” is anticipated to contribute $5,293 million USD in revenue, making up 27.50% of the market share. This distribution highlights the significant role of stents and guidewires in driving the overall growth of the Nitinol Medical Devices market.
Table Global Nitinol Medical Devices Market Size and Share by Type in 2024
Type | Market Size in 2024 (M USD) | Market Share in 2024 (%) |
|---|---|---|
Stents | 7864 | 40.87% |
Guidewires | 6086 | 31.63% |
Others | 5293 | 27.50% |
5 Global Nitinol Medical Devices Market Analysis by Application
In 2024, the global Nitinol Medical Devices market is projected to generate a total revenue of $19,242 million USD across various applications. The largest segment is expected to be the Vascular application, with a revenue of $12,382 million USD, accounting for 64.35% of the total market share. The Orthopedic & Dental segment is forecasted to contribute $4,349 million USD, representing 22.60% of the market. The remaining segment, classified as “Others,” is anticipated to bring in $2,512 million USD in revenue, holding a market share of 13.05%. This breakdown underscores the dominant role of vascular applications in the Nitinol Medical Devices market, driven by the increasing demand for minimally invasive procedures and the material’s suitability for cardiovascular devices.
Table Global Nitinol Medical Devices Market Size and Share by Application in 2024
Application | Market Size in 2024 (M USD) | Market Share in 2024 (%) |
|---|---|---|
Vascular | 12382 | 64.35% |
Orthopedic & Dental | 4349 | 22.60% |
Others | 2512 | 13.05% |
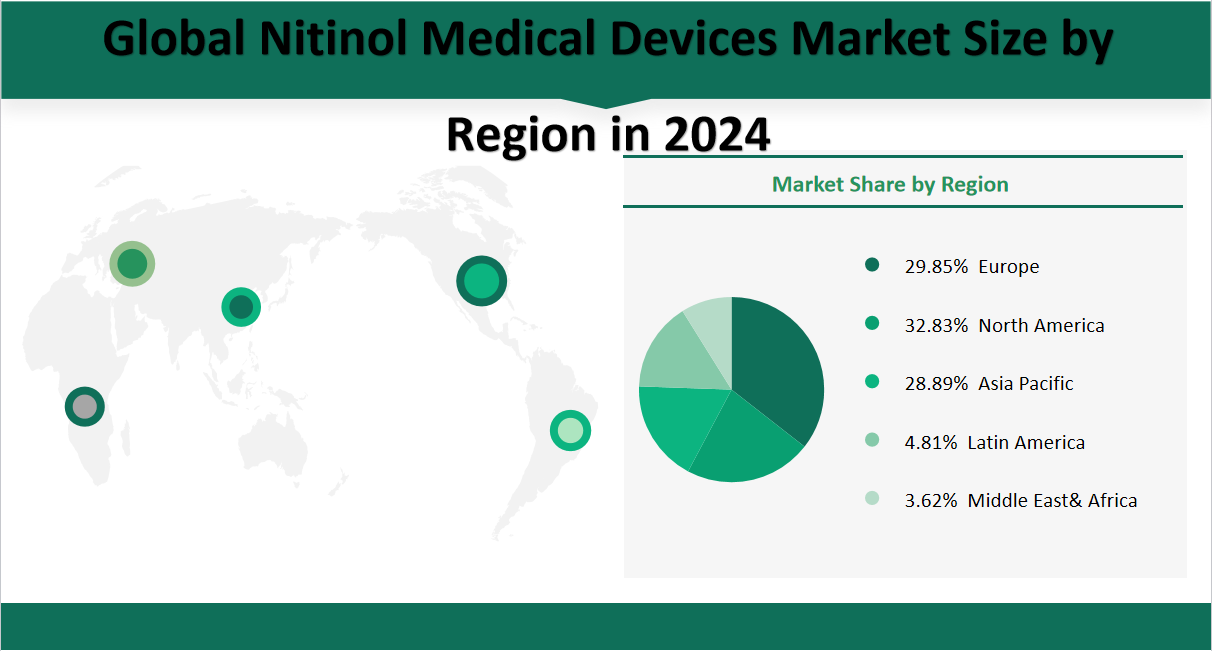
6 Global Nitinol Medical Devices Market Analysis by Region
In 2024, the global Nitinol Medical Devices market is forecasted to generate a total revenue of $19,242 million USD. Regionally, North America is expected to remain the largest market, with a revenue of $6,318 million USD, accounting for 32.83% of the global market share. Europe follows closely, contributing $5,744 million USD or 29.85% of the total market. The Asia-Pacific region is anticipated to be the fastest-growing market, with a revenue of $5,559 million USD and a market share of 28.89%. Latin America is projected to generate $925 million USD, representing 4.81% of the global market, while the Middle East & Africa is expected to contribute $697 million US*, holding a 3.62% market share. This regional distribution highlights the continued dominance of North America and Europe, while the Asia-Pacific region is poised for significant growth due to increasing healthcare expenditure and demand for advanced medical technologies.
Figure Global Nitinol Medical Devices Market Share by Region in 2024
7 Top 3 Companies of Global Nitinol Medical Devices Market
7.1 Abbott
Company Introduction and Business Overview:
Abbott Laboratories, commonly known as Abbott, is a global healthcare company founded in 1888 and headquartered in the United States. The company is renowned for its wide range of life-changing technologies that span diagnostics, medical devices, nutritionals, and branded generic medicines. Abbott’s commitment to improving healthcare outcomes is evident through its innovative products and solutions designed to address various medical needs across the globe. With over 109,000 employees, Abbott serves people in more than 160 countries, making it a significant player in the global healthcare industry.
Products Offered:
Abbott’s Nitinol Medical Devices portfolio includes a variety of products designed to enhance minimally invasive procedures. One of their flagship products is the SUPERA™ Peripheral Stent, which is indicated for use in the superficial femoral artery (SFA) and the proximal popliteal artery. This stent is engineered with a unique interwoven wire technology, offering unmatched clinical outcomes across varied lesion complexities and lengths. Key features include high compression resistance, low chronic outward force, and unparalleled flexibility, making it highly effective in treating peripheral artery disease.
In addition to stents, Abbott also offers a range of Nitinol-based guidewires and other medical devices that leverage the material’s superelasticity and shape memory properties. These products are designed to improve patient outcomes by providing greater precision, flexibility, and durability compared to traditional materials.
Sales Revenue in the Latest Year:
In the latest year, Abbott’s Nitinol Medical Devices revenue was reported at $2,355 million USD. This figure reflects Abbott’s strong market position and its continuous efforts to innovate and expand its product offerings. The company’s focus on research and development, coupled with strategic acquisitions and partnerships, has enabled it to maintain a robust growth trajectory in the Nitinol Medical Devices market.
7.2 Medtronic
Company Introduction and Business Overview:
Medtronic plc is a leading global medical technology company founded in 1949 by Earl Bakken and Palmer Hermundslie in Minneapolis, Minnesota. The company has since grown to become a major player in the medical device industry, with operations and executive headquarters in Fridley, Minnesota. Medtronic is dedicated to alleviating pain, restoring health, and extending life through its innovative medical technologies. The company’s product portfolio spans a wide range of therapeutic areas, including cardiovascular, spinal, neurological, and diabetes management.
Products Offered:
Medtronic’s Nitinol Medical Devices portfolio includes products such as the Nitrex Guidewire, which is designed for use in both peripheral and coronary vasculature. The guidewire features a gently tapered, continuous Nitinol core that provides flexibility and kink resistance. Additionally, the gold-plated tungsten coiling enhances radiopacity for better fluoroscopic visualization, while the mandril design allows for precise 1:1 torque control. These features make the Nitrex Guidewire highly effective in navigating complex anatomical structures during minimally invasive procedures.
Medtronic also offers a variety of other Nitinol-based devices, including stents and surgical instruments, designed to leverage the material’s unique properties for improved patient outcomes. The company’s focus on innovation and quality has positioned it as a trusted provider of medical solutions worldwide.
Sales Revenue in the Latest Year:
Medtronic’s Nitinol Medical Devices revenue reached $1,740 million USD. This revenue figure highlights Medtronic’s strong market presence and its commitment to developing advanced medical technologies. The company’s continuous investment in research and development, along with its strategic market expansion efforts, have contributed to its sustained growth in the Nitinol Medical Devices market.
7.3 Edwards Lifesciences
Company Introduction and Business Overview:
Edwards Lifesciences, founded in 1958 and headquartered in Irvine, California, is a leading medical technology company specializing in artificial heart valves and hemodynamic monitoring. The company is renowned for its innovative products, such as the SAPIEN transcatheter aortic heart valve, which is designed to provide minimally invasive treatment options for patients with aortic stenosis. Edwards Lifesciences operates manufacturing facilities in multiple locations, including the United States, Costa Rica, the Dominican Republic, Puerto Rico, and Singapore. The company’s dedication to improving patient outcomes through advanced medical technologies has solidified its position as a key player in the global healthcare industry.
Products Offered:
Edwards Lifesciences’ Nitinol Medical Devices portfolio includes products designed to address various cardiovascular conditions. One of their notable products is the Pulsar-18 Stent, which is indicated for use in patients with atherosclerotic disease of the femoral and infrapopliteal arteries. The stent features a Nitinol material with a strut thickness of 140 μm and a strut width of 85 μm, providing optimal flexibility and vessel conformity. The stent is also coated with proBIO (amorphous silicon carbide) to enhance biocompatibility and reduce the risk of complications.
In addition to stents, Edwards Lifesciences offers a range of other Nitinol-based devices, including guidewires and surgical instruments, designed to leverage the material’s superelasticity and shape memory properties. These products are aimed at improving patient outcomes by providing minimally invasive treatment options and reducing recovery times.
Sales Revenue in the Latest Year:
Edwards Lifesciences reported a Nitinol Medical Devices revenue of $1,329 million USD. This revenue figure reflects the company’s strong market position and its continuous efforts to innovate and expand its product offerings. Edwards Lifesciences’ focus on developing advanced cardiovascular solutions has positioned it as a trusted provider of medical devices worldwide.

