1 Global Enterovirus Vaccine Market Outlook
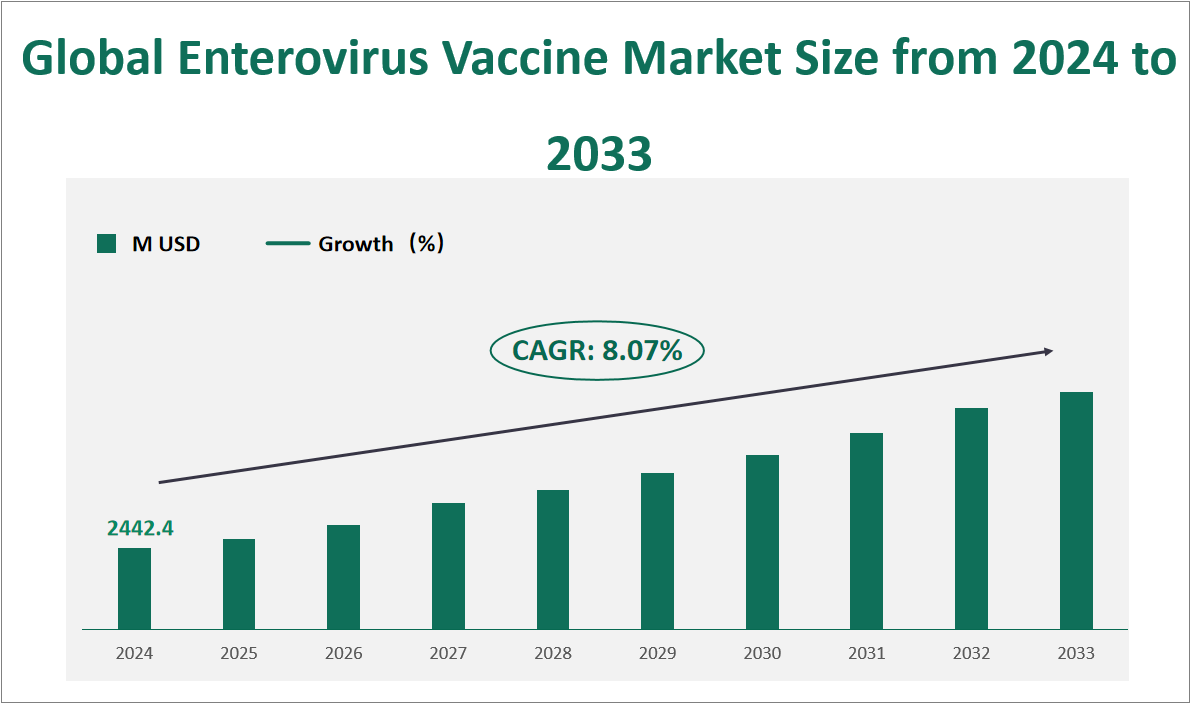
The global Enterovirus Vaccine market is projected to exhibit substantial growth in the coming years, with a CAGR of 8.07% from 2024 to 2033, reaching a total market size of $2442.4 million USD in 2024. Enteroviruses are a genus of positive-sense single-stranded RNA viruses that cause a variety of diseases in humans and animals. They are classified into several groups, including polioviruses, Coxsackie A viruses, Coxsackie B viruses, and echoviruses. Among these, Enterovirus 71 (EV71) has gained significant attention due to its association with severe neurological complications and hand, foot, and mouth disease (HFMD) in children. Vaccines against enteroviruses, particularly EV71, have become crucial in preventing outbreaks and reducing the incidence of severe infections.
The development of enterovirus vaccines involves complex processes, including the use of inactivated whole-virus vaccines, live-attenuated virus vaccines, virus-like particle (VLP) vaccines, and recombinant protein vaccines. These vaccines aim to elicit strong immune responses and produce neutralizing antibodies, providing protection against enterovirus infections. The primary strategy for vaccine development involves using viral structural proteins as immunogens to stimulate host immunogenicity.
Figure Global Enterovirus Vaccine Market Size and Growth Rate (2024-2033)
2 Enterovirus Vaccine Market Growth Drivers and Constraints
The growth of the global Enterovirus Vaccine market is driven by several key factors. The increasing incidence of hand-foot-mouth disease (HFMD) and other enterovirus-related infections has heightened the demand for effective vaccines. HFMD, caused by viruses such as EV71 and Coxsackieviruses, has led to significant outbreaks in the Asia-Pacific region, highlighting the need for preventive measures. Additionally, the lack of specific treatments for enterovirus infections makes vaccination the most effective strategy to control these diseases.
Technological advancements in vaccine development have also played a crucial role in driving market growth. Innovations in vaccine technology, such as the development of inactivated EV71 vaccines, have demonstrated high efficacy and safety profiles, further boosting market demand. The ongoing research and development efforts by major pharmaceutical companies and research institutions are aimed at improving vaccine efficacy and expanding coverage to multiple serotypes.
However, the market faces several challenges that may limit its growth. One of the primary challenges is the high cost and complexity of vaccine development. The process from research to licensed product can take up to 15 years, involving significant investment in research, clinical trials, and regulatory approvals. Additionally, the storage and distribution of vaccines require specialized equipment and monitoring systems, adding to the overall costs.
Regulatory hurdles and the need for harmonized vaccine standards also pose significant challenges. Ensuring the safety and efficacy of vaccines across different regions requires stringent regulatory oversight. The World Health Organization (WHO) plays a crucial role in developing quality control and assessment standards for enterovirus vaccines, but achieving broad approval remains a complex process.
3 Enterovirus Vaccine Market Innovations and M&A Activities
Technological innovation has been a cornerstone of the Enterovirus Vaccine market. The development of inactivated vaccines, such as the EV71 vaccine, has been a significant milestone. These vaccines have shown high efficacy in preventing severe cases of HFMD and have been widely adopted in regions with high infection rates. The use of advanced biotechnological techniques, such as genetic engineering and cell-based production systems, has enabled the development of more effective and safer vaccines.
Corporate mergers and acquisitions have also played a significant role in shaping the market landscape. Major players such as Sanofi Pasteur, GSK, and Sinovac Biotech have been actively involved in strategic partnerships and acquisitions to enhance their vaccine portfolios. For instance, Sanofi Pasteur’s acquisition of Translate Bio aims to advance the deployment of mRNA technology in vaccine development. Similarly, GSK’s acquisition of Affinivax, Inc. is focused on developing next-generation pneumococcal vaccines, which could have broader applications in the enterovirus vaccine market.
These strategic moves not only strengthen the companies’ positions in the market but also drive innovation and the development of new vaccine technologies. The collaboration between research institutions and pharmaceutical companies has been crucial in accelerating the development and commercialization of enterovirus vaccines. The ongoing efforts to develop multivalent vaccines that provide broad-spectrum protection against multiple serotypes represent the next frontier in vaccine innovation.
In conclusion, the global Enterovirus Vaccine market is poised for significant growth driven by increasing demand for effective vaccines, technological advancements, and strategic corporate activities. However, the market must overcome challenges related to high development costs, regulatory hurdles, and the need for harmonized standards to ensure the widespread availability and adoption of these vaccines.
4 Global Enterovirus Vaccine Market Analysis by Type
In 2024, the global Enterovirus Vaccine market is projected to generate a total revenue of US2,442.4million. This revenue is divided into two main types of vaccines: Human Enterovirus 71 and Polioviruses. Specifically, the revenue from Human Enterovirus 71 vaccines is expected to reach US 624.8 million, accounting for approximately 25.58% of the total market revenue. Meanwhile, the revenue from Poliovirus vaccines is anticipated to be US$ 1817.6 million, representing 74.42% of the total market revenue. This distribution highlights the significant contribution of Poliovirus vaccines to the overall market, while also indicating the growing importance of Human Enterovirus 71 vaccines in addressing specific enterovirus-related diseases.
Table Global Enterovirus Vaccine Market Size and Share by Type in 2024
Type | Market Size in 2024 (M USD) | Market Share in 2024 (%) |
|---|---|---|
Human Enterovirus 71 | 624.8 | 25.58% |
Polioviruses | 1817.6 | 74.42% |
5 Global Enterovirus Vaccine Market Analysis by Application
In 2024, the global Enterovirus Vaccine market is expected to generate a total revenue of US 2,442.4 million, with the revenue distributed across different application areas. Specifically, the revenue from vaccines used in hospitals is projected to be US 1875.5 million, accounting for 76.79% of the total market revenue. This indicates that hospitals remain the primary application area for enterovirus vaccines. Meanwhile, the revenue from vaccines used in clinics is expected to reach US 473 million, representing 19.37%. 93.8 million, is attributed to other applications. This distribution highlights the dominant role of hospitals in the administration of enterovirus vaccines, while also noting the significant contribution from clinics and other smaller application areas.
Table Global Enterovirus Vaccine Market Size and Share by Application in 2024
Application | Market Size in 2024 (M USD) | Market Share in 2024 (%) |
|---|---|---|
Hospital | 1875.5 | 76.79% |
Clinic | 473.0 | 19.37% |
Others | 93.8 | 3.84% |
6 Global Enterovirus Vaccine Market Analysis by Region
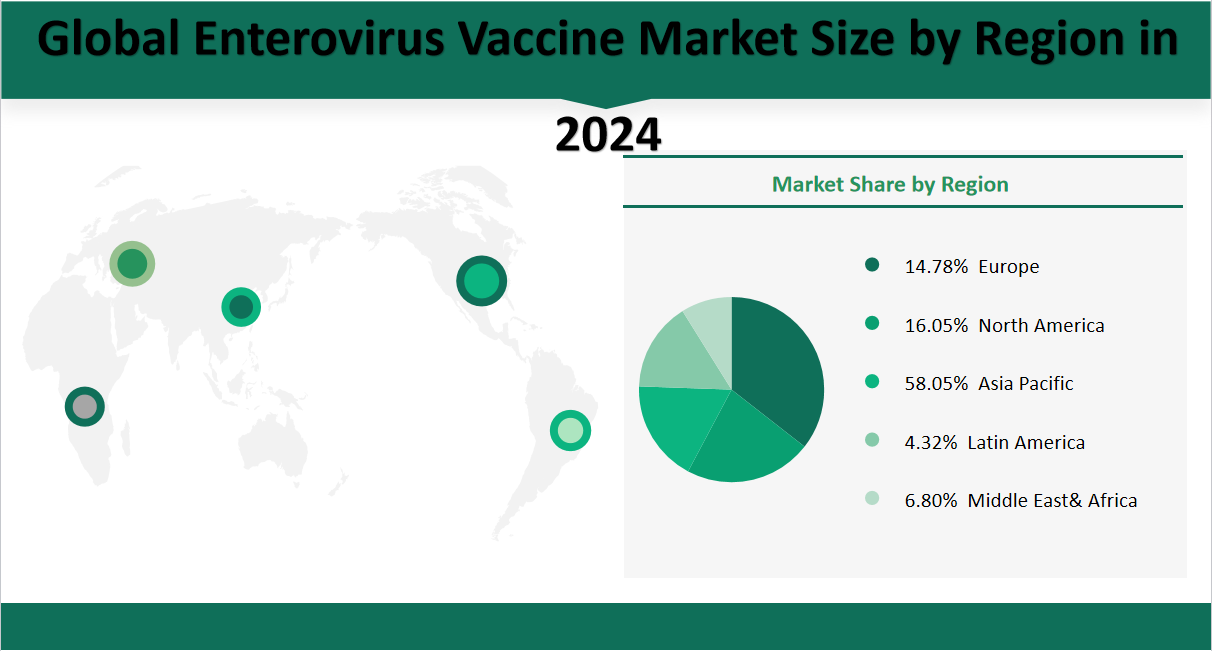
In 2024, the global Enterovirus Vaccine market is projected to generate a total revenue of US 2,217.5 million, with significant contributions from various regions. North America is expected to contribute US 391.9 million, representing 16.05% of the global revenue. The Asia-Pacific region is anticipated to lead with US 1417.8 million, accounting for 58.05%. Europe is expected to contribute US 361.1 million, or 14.78% of the total revenue. Latin America is expected to generate US 105.5 million, making up 4.32%. Middle East and Africa is expected to contribute US 166.1 million, or 6.8% of the total. These figures highlight the dominant role of the Asia-Pacific region in the global Enterovirus Vaccine market, with North America and Europe also playing significant roles.
Figure Global Enterovirus Vaccine Market Share by Region in 2024
7 Top 3 Companies of Global Enterovirus Vaccine Market
7.1 Sanofi Pasteur
Company Introduction and Business Overview:
Sanofi Pasteur is the vaccines division of Sanofi, a global healthcare leader based in France. Established in 2004, Sanofi Pasteur is the largest company in the world dedicated entirely to vaccines. The company has a rich history in vaccine development, with a commitment to improving public health through innovative immunization solutions. Sanofi Pasteur operates in over 100 countries and has a diverse portfolio of vaccines that protect against various infectious diseases, including influenza, meningitis, and polio.
Products Offered:
Sanofi Pasteur offers a range of enterovirus vaccines, with a notable focus on the inactivated poliovirus vaccine (IPV) and the enterovirus 71 (EV71) vaccine. The IPV is crucial for preventing poliomyelitis, a disease that can lead to paralysis and even death. The EV71 vaccine is designed to protect against hand, foot, and mouth disease (HFMD), which is caused by enterovirus infections, particularly in children. Sanofi Pasteur’s vaccines are developed using advanced technologies and adhere to stringent safety and efficacy standards, ensuring high levels of immunogenicity.
Sales Revenue in the Latest Year:
Sanofi Pasteur reported a revenue of US$ 751.2 million from its enterovirus vaccine portfolio. This figure reflects the company’s strong market presence and its pivotal role in the global fight against enterovirus-related diseases. Sanofi Pasteur’s continuous investment in research and development has enabled it to maintain a competitive edge and expand its vaccine offerings to meet the growing demand.
7.2 Chinese Academy of Medical Sciences
Company Introduction and Business Overview:
The Chinese Academy of Medical Sciences (CAMS) is a leading research institution in China, established in 1955. It focuses on medical research, education, and public health initiatives. CAMS is dedicated to advancing healthcare through scientific research and innovation, particularly in the field of infectious diseases. The academy collaborates with various international organizations and plays a crucial role in vaccine development and public health policy in China and beyond.
Products Offered:
CAMS has developed several enterovirus vaccines, including the inactivated EV71 vaccine and the Sabin strain inactivated polio vaccine. The EV71 vaccine is designed to prevent HFMD, which has become a significant public health concern in China. The vaccine has shown high efficacy in clinical trials and is now widely used in vaccination campaigns across the country. Additionally, CAMS produces the inactivated polio vaccine, which is essential for controlling poliovirus transmission and preventing outbreaks.
Sales Revenue in the Latest Year:
The Chinese Academy of Medical Sciences reported a revenue of US$ 502.1 million from its enterovirus vaccine sales. This revenue underscores the academy’s significant contribution to the global enterovirus vaccine market and its commitment to improving public health through effective vaccination programs. CAMS continues to invest in research and development to enhance its vaccine offerings and address emerging health challenges.
7.3 GSK (GlaxoSmithKline)
Company Introduction and Business Overview:
GSK, or GlaxoSmithKline, is a British multinational pharmaceutical company headquartered in London, UK. Established in 2000, GSK is one of the world’s largest pharmaceutical companies, with a strong focus on research and development in various therapeutic areas, including vaccines, respiratory diseases, and infectious diseases. GSK is committed to improving the quality of human life by enabling people to do more, feel better, and live longer.
Products Offered:
GSK offers a variety of vaccines, including those targeting enteroviruses. One of its key products is the Boostrix IPV vaccine, which provides protection against diphtheria, tetanus, pertussis, and poliomyelitis. This vaccine is crucial for maintaining immunity in adults and children who have previously been vaccinated. GSK is also involved in the development of vaccines targeting enterovirus infections, particularly those associated with HFMD.
Sales Revenue in the Latest Year:
GSK reported a revenue of US$ 374.6 million from its enterovirus vaccine sales. This revenue reflects GSK’s strong market position and its ongoing commitment to vaccine innovation and public health. The company continues to invest in research and development to expand its vaccine portfolio and address the evolving needs of the global healthcare landscape.

