1 Global Bladder Cancer Market Size (Value) and CAGR (2024-2033)
In 2024, the global Bladder Cancer market was valued at USD 6.79 Billion, with a CAGR of 18.6% from 2024 to 2033.
Bladder cancer is a disease in which cells in the urinary bladder start growing abnormally and uncontrollably. The main types of bladder cancer are transitional cell carcinomas (also known as urothelial carcinomas), which begins in the cells that line the inside of the bladder. Rarer bladder cancers include squamous cell carcinomas (originating from flat-shaped cells), adenocarcinomas (from mucus-secreting gland cells), small cell carcinoma (from nerve-like neuroendocrine cells) and sarcomas (from the bladder’s muscle cells).
Figure Global Bladder Cancer Market Size (M USD) and CAGR 2024-2033

2 Bladder Cancer Market Drivers
Increasing Prevalence of Bladder Cancer
Bladder cancer is one of the most common cancers globally, particularly among the aging population. According to the World Cancer Research Fund, bladder cancer ranks as the 6th most common cancer in men and the 17th most common in women. The International Agency for Research on Cancer (IARC) reported that in 2020, there were 573,278 new cases diagnosed worldwide.
This high incidence rate is a significant driver of the market, as it creates a continuous demand for effective diagnostic and treatment options. Additionally, the aging population is a crucial factor, with approximately 90% of bladder cancer cases diagnosed in individuals over the age of 55. As the global population continues to age, the prevalence of bladder cancer is expected to rise, further fueling market growth.
Lifestyle Factors: Smoking and Tobacco Consumption
Smoking is a major risk factor for bladder cancer, accounting for nearly half of all cases. Smokers are three times more likely to develop bladder cancer compared to non-smokers. Despite a decline in smoking rates globally, the total number of smokers has increased due to population growth. In 2019, there were an estimated 155 million smokers aged 15 to 24, representing 20.1% of young men and 5.0% of young women globally. The United States alone has about 37.8 million adult smokers. The high prevalence of smoking, especially among men, significantly contributes to the rising incidence of bladder cancer, thereby driving the demand for treatment and diagnostic services.
3 Bladder Cancer Market Restraints
Strict Regulatory Oversight
Bladder cancer drugs and treatments are subject to stringent regulations by national and international health authorities. These regulations are designed to ensure the safety and efficacy of medical products but can also create barriers to market entry. For example, the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) require extensive clinical trials and rigorous testing before approving new drugs.
This process can be time-consuming and costly for pharmaceutical companies. Additionally, the approval process may vary across different regions, requiring companies to navigate multiple regulatory frameworks. For instance, the approval of avelumab for bladder cancer treatment required extensive clinical trials and regulatory submissions in both the United States and Europe.
High Risk of Drug Development Failure
Developing new drugs for bladder cancer is a complex and uncertain process. Clinical trials are often lengthy and expensive, with many potential pitfalls. Factors such as patient recruitment, eligibility criteria, and competition from other clinical trials can delay the development process. Moreover, even if a drug shows promise in early-stage trials, it may fail to demonstrate sufficient safety and efficacy in later stages.
4 Global Bladder Cancer Market Size and Share by Type in 2024
Non-Muscle-Invasive Bladder Cancer (NMIBC) is the most prevalent form of bladder cancer, accounting for approximately 82.17% of the total market share in 2024. This type of cancer originates in the cells lining the inside of the bladder and does not invade the muscle layer of the bladder wall. NMIBC is further classified into subtypes such as papillary urothelial neoplasm of low malignant potential (PUNLMP), low-grade papillary carcinoma, and high-grade papillary carcinoma.
The treatment for NMIBC often includes minimally invasive procedures like transurethral resection of the bladder tumor (TURBT) and intravesical therapies, including Bacillus Calmette-Guérin (BCG) or chemotherapy agents. These treatments aim to remove the tumor and prevent its recurrence while preserving the bladder’s function. The market size for NMIBC in 2024 is estimated at $5,577.03 million, reflecting the significant demand for effective diagnostic and treatment options driven by its high prevalence.
Muscle-Invasive Bladder Cancer (MIBC), on the other hand, is a more aggressive form of the disease, representing about 17.83% of the total market share in 2024. MIBC invades the muscle layer of the bladder wall and has a higher propensity to metastasize to other parts of the body. This type of cancer is often diagnosed at a later stage, making it more challenging to treat.
The standard treatment for MIBC includes radical cystectomy (surgical removal of the bladder), chemotherapy, and in some cases, radiation therapy. Advances in immunotherapy and targeted therapies are also being explored for MIBC, driving innovation in this segment. The market size for MIBC in 2024 is projected at $1,209.97 million. This segment’s growth is influenced by the need for advanced treatments that can improve survival rates and quality of life for patients.
Table Global Bladder Cancer Market Size and Share by Type in 2024
Type | Market Size (M USD) 2024 | Market Share 2024 |
Non-Muscle-Invasive Bladder Cancer | 5577.03 | 82.17% |
Muscle-Invasive Bladder Cancer | 1209.97 | 17.83% |
5 Global Bladder Cancer Market Size and Share by Application in 2024
The bladder cancer market is segmented by application into hospitals, clinics, and other settings, each serving unique roles in the treatment and management of the disease. Hospitals dominate the market, with a projected value of $3,293.93 million in 2024. They are the primary centers for comprehensive care, offering advanced diagnostic tools, surgical interventions, and multidisciplinary treatment plans. Hospitals are often equipped with state-of-the-art facilities and specialized oncology departments, making them the go-to option for patients with advanced or complex cases of bladder cancer.
Clinics, including specialized oncology and urology centers, contribute significantly to the market with a projected value of $2,005.36 million in 2024. These settings focus on early detection, minimally invasive procedures, and follow-up care. Clinics play a crucial role in patient education and preventive care, often being the first point of contact for individuals experiencing symptoms or undergoing routine screenings. Their accessibility and specialized services make them essential in managing the early stages of bladder cancer.
Table Global Bladder Cancer Market Size and Share by Application in 2024
Application | Market Size (M USD) 2024 | Market Share 2024 |
Hospital | 3293.93 | 48.53% |
Clinic | 2005.36 | 29.55% |
Others | 1487.70 | 21.92% |
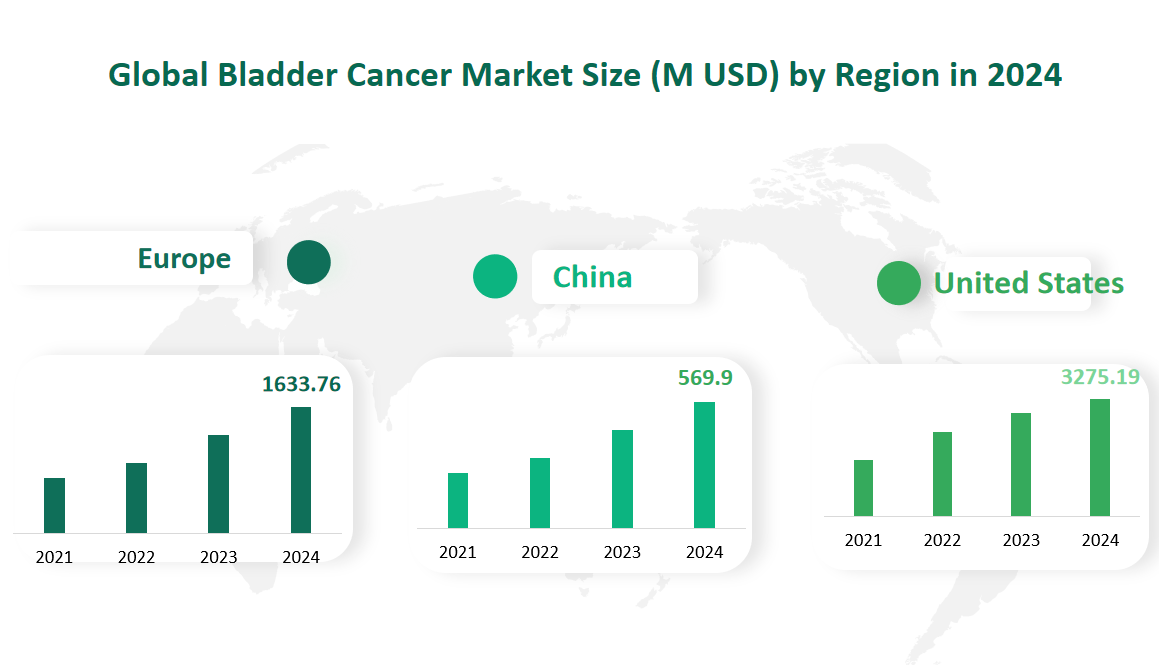
6 Global Bladder Cancer Market Size by Region in 2024
United States holds the largest share of the market, with a projected value of $3275.19 million in 2024. The region’s dominance is attributed to advanced healthcare systems, significant investment in research and development, and high demand for cutting-edge treatments. The U.S. market is particularly driven by the aging population, increased awareness of bladder cancer, and favorable reimbursement policies that support access to advanced therapies.
Europe follows closely, with a projected market value of $1,633.76 million in 2024. European countries, such as Germany, the UK, France, Italy, and Spain, contribute significantly to the market. Europe’s strength lies in its focus on healthcare quality, innovation, and early detection programs. The region is known for its robust clinical research infrastructure and adoption of advanced diagnostic and treatment technologies. Government policies that promote comprehensive cancer care further support market growth.
China is experiencing rapid growth, with a projected market value of $569.9 million in 2024. The region’s growth is fueled by rising awareness of bladder cancer, an aging population, and expanding insurance coverage. Additionally, government initiatives to enhance cancer care and promote early detection are contributing to the region’s dynamic market landscape.
Figure Global Bladder Cancer Market Size by Region in 2024
7 Major Players in Global Bladder Cancer Market
7.1 Merck & Co. Inc.
Company Profile: Merck & Co. Inc., known as Merck outside the United States and Canada, is a leading global pharmaceutical company with a history dating back to 1891. Headquartered in Kenilworth, New Jersey, USA, Merck is dedicated to inventing for life by providing innovative medicines and vaccines for some of the world’s most challenging diseases. The company operates in over 140 countries, partnering with customers to deliver health solutions that improve lives.
Business Overview: Merck’s business is centered around its commitment to advancing science and improving healthcare outcomes. The company’s portfolio includes prescription drugs, vaccines, biotherapeutics, and animal health products. Merck has been at the forefront of research in oncology, cardiometabolic diseases, immunology, and infectious diseases. The company’s dedication to innovation and accessibility has led to the development of several life-changing medicines, including those for bladder cancer.
Product Profiles: One of Merck’s key products in the bladder cancer market is KEYTRUDA (pembrolizumab), a humanized monoclonal antibody that targets the PD-1 receptor. KEYTRUDA is indicated for the treatment of high-risk, non-muscle-invasive bladder cancer (NMIBC) in patients who have failed Bacillus Calmette-Guérin (BCG) therapy. The drug has shown significant efficacy in clinical trials, making it a preferred choice for many patients and healthcare providers.
Recent Financial Performance: In the most recent year, Merck & Co. Inc. reported a revenue of $1141.59 million from its bladder cancer-related products.
7.2 Bristol-Myers Squibb Company
Company Profile: Bristol-Myers Squibb Company, established in 1887, is a global biopharmaceutical company headquartered in New York, USA. The company is renowned for its focus on discovering, developing, and delivering innovative medicines that address serious diseases. Bristol-Myers Squibb’s commitment to advancing science and improving patient outcomes has led to the development of several groundbreaking therapies across various therapeutic areas.
Business Overview: Bristol-Myers Squibb’s business strategy is centered on leveraging translational medicine and data analytics to deliver the right medicine to the right patient at the right time. The company’s portfolio includes treatments for oncology, cardiovascular diseases, immunoscience, and fibrosis. Bristol-Myers Squibb is dedicated to improving patient access to medical innovations through collaborations with academic institutions, biotechnology companies, and healthcare providers.
Product Profiles: Bristol-Myers Squibb’s key product in the bladder cancer market is OPDIVO (nivolumab), an immune checkpoint inhibitor that targets the PD-1 receptor. OPDIVO is indicated for the treatment of locally advanced or metastatic urothelial carcinoma, including bladder cancer, in patients who have previously received platinum-containing chemotherapy. The drug has demonstrated significant clinical benefits, making it a valuable addition to the treatment landscape for bladder cancer.
Recent Financial Performance: In the most recent year, Bristol-Myers Squibb Company reported a revenue of $652.29 million from its bladder cancer-related products.
7.3 F. Hoffmann-La Roche AG
Company Profile: F. Hoffmann-La Roche AG, commonly known as Roche, is a global pioneer in pharmaceuticals and diagnostics, founded in 1896 and headquartered in Basel, Switzerland. Roche is committed to advancing science to improve people’s lives through personalized healthcare. The company’s combined strengths in medicines and diagnostics have made it a leader in the industry, with a focus on delivering the right treatment to every patient in the best possible way.
Business Overview: Roche’s business is characterized by its dedication to innovation and scientific excellence. The company’s portfolio includes treatments for oncology, immunology, infectious diseases, ophthalmology, and central nervous system disorders. Roche is also a leader in in vitro diagnostics and tissue-based cancer diagnostics. The company’s commitment to improving patient outcomes through personalized healthcare has led to the development of several life-changing medicines.
Product Profiles: Roche’s key product in the bladder cancer market is TECENTRIQ (atezolizumab), an immune checkpoint inhibitor that targets the PD-L1 receptor. TECENTRIQ is indicated for the treatment of locally advanced or metastatic urothelial carcinoma, including bladder cancer, in patients who are ineligible for cisplatin-containing chemotherapy. The drug has demonstrated significant clinical benefits, making it a preferred choice for many patients and healthcare providers.
Recent Financial Performance: In the most recent year, F. Hoffmann-La Roche AG reported a revenue of $484.56 million from its bladder cancer-related products.

