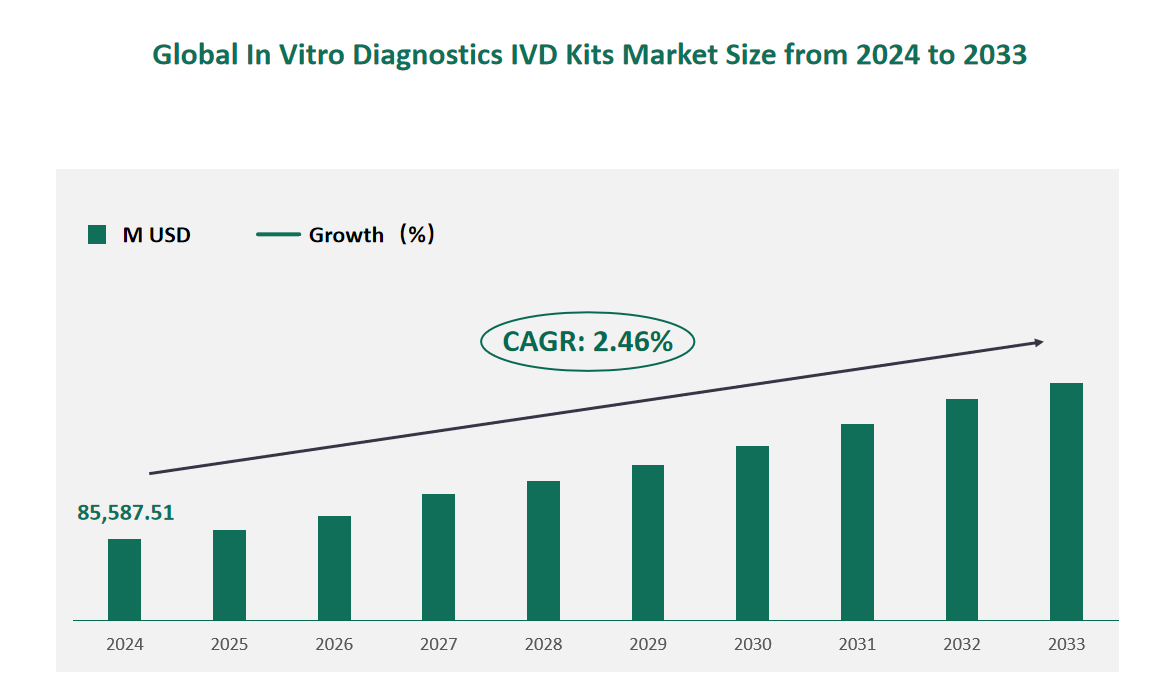
1 Global In Vitro Diagnostics IVD Kits Market Size (Value) and CAGR (2024-2033)
In 2024, the global In Vitro Diagnostics IVD Kits market was valued at USD 85,587.51 million, with a CAGR of 2.46% from 2024 to 2033.
In vitro diagnostics (IVD) are tests done on samples such as blood or tissue that have been taken from the human body. In vitro diagnostics can detect diseases or other conditions, and can be used to monitor a person’s overall health to help cure, treat, or prevent diseases. In vitro diagnostic (IVD) test kit consists of materials used to determine the outcome of a given test. This report studies the global In Vitro Diagnostics IVD Kits market.
Figure Global In Vitro Diagnostics IVD Kits Market Size (M USD) and CAGR 2024-2033
2 In Vitro Diagnostics IVD Kits Market Drivers
One of the most prominent driving factors is the growing prevalence of chronic diseases such as diabetes, cardiovascular diseases, and cancer. These conditions require regular monitoring and early detection to manage effectively. IVD kits play a crucial role in diagnosing and monitoring these diseases, enabling healthcare providers to offer timely and appropriate treatments.
Advancements in medical technology have significantly impacted the IVD Kits market. Innovations in molecular diagnostics, point-of-care testing (POCT), and automation have enhanced the accuracy, speed, and accessibility of diagnostic tests. For instance, the development of real-time PCR (polymerase chain reaction) and next-generation sequencing technologies has revolutionized the detection and analysis of pathogens and genetic markers. These technological advancements not only improve diagnostic capabilities but also reduce the time and cost associated with testing, making IVD kits more accessible and efficient.
The global population is aging, with a significant increase in the number of individuals over 60 years old. Older adults are more susceptible to chronic diseases and require more frequent medical interventions. This demographic shift has led to an increased demand for healthcare services, including diagnostic testing. IVD kits are essential tools in managing the health of the aging population, as they enable early detection and effective treatment of various conditions. The rising healthcare demand driven by an aging population is a significant factor contributing to the growth of the IVD Kits market.
3 In Vitro Diagnostics IVD Kits Market Restraints
One of the most significant challenges in the IVD Kits market is the complex regulatory environment. IVD kits are subject to stringent regulations by authorities such as the U.S. Food and Drug Administration (FDA) and the European Union’s In Vitro Diagnostic Regulation (IVDR). These regulations ensure the safety and efficacy of diagnostic products but also impose rigorous testing, approval, and compliance requirements. The lengthy and costly process of obtaining regulatory approvals can delay the introduction of new products and limit market entry for smaller companies. Additionally, the need for continuous compliance with evolving regulations adds to the operational burden of manufacturers.
The IVD Kits market is characterized by high entry barriers, making it difficult for new players to establish a foothold. The initial investment required for research and development, manufacturing infrastructure, and regulatory compliance is substantial. Furthermore, the market is dominated by a few large players with extensive experience, established distribution networks, and strong brand recognition. New entrants must overcome these challenges to compete effectively, which often requires significant financial resources and technical expertise.
The IVD Kits market is highly competitive, with several major players vying for market share. Companies such as Roche Diagnostics, Abbott Laboratories, and Danaher Corporation dominate the market, leveraging their extensive product portfolios, advanced technologies, and global reach. Intense competition can lead to aggressive pricing strategies, reduced profit margins, and the need for continuous innovation to stay ahead. Smaller companies often struggle to compete with these giants, limiting their growth opportunities and market penetration.
4 Global In Vitro Diagnostics IVD Kits Market Size by Type in 2024
Blood is the most commonly used sample type in IVD kits. It is utilized for a wide range of diagnostic tests, including those for infectious diseases, diabetes, cardiology, and oncology. Blood tests are essential for detecting biomarkers, pathogens, and other indicators of health conditions. In 2024, the blood segment is expected to generate a revenue of 57,846.30 million USD. This segment’s dominance is attributed to its versatility and the critical role it plays in diagnosing and monitoring various diseases.
Urine tests are another significant segment in the IVD Kits market. These tests are primarily used for detecting urinary tract infections, kidney diseases, and other conditions. Urine samples are non-invasive and easy to collect, making them a popular choice for routine health check-ups and disease monitoring. In 2024, the urine segment is projected to reach a market value of 5,544.39 million USD. The growth of this segment is driven by the increasing prevalence of chronic kidney diseases and the need for early detection and management of urinary conditions.
Stool tests are used to detect gastrointestinal infections, parasitic diseases, and other digestive disorders. These tests are crucial for diagnosing conditions such as inflammatory bowel disease (IBD) and colorectal cancer. In 2024, the stool segment is expected to contribute 1,802.17 million USD to the global IVD Kits market. The demand for stool tests is growing due to the increasing awareness of gastrointestinal health and the need for accurate diagnostic tools in this area.
Tissue cell tests are essential for diagnosing and monitoring various cancers and other tissue-related conditions. These tests involve the analysis of tissue samples obtained through biopsies or other procedures. In 2024, the tissue cell segment is projected to generate a revenue of 16,531.14 million USD. The growth of this segment is driven by advancements in molecular diagnostics and the increasing demand for precise diagnostic tools in oncology and other fields.
Table Global In Vitro Diagnostics IVD Kits Market Size by Type in 2024
Type | Market Size (M USD) 2024 |
Blood | 57846.30 |
Urine | 5544.39 |
Stool | 1802.17 |
Tissue Cells | 16531.14 |
Others | 3863.50 |
5 Global In Vitro Diagnostics IVD Kits Market Size by Application in 2024
Infectious diseases remain a significant global health concern, driving the demand for IVD kits in this application segment. These kits are essential for the rapid detection and management of various pathogens, including viruses, bacteria, and fungi. In 2024, the infectious diseases segment is expected to generate a revenue of 50,478.96 million USD. This segment’s growth is fueled by the ongoing need for accurate and timely diagnostic tools, especially in the context of emerging infectious diseases and pandemics.
Diabetes is a chronic condition that requires regular monitoring and management. IVD kits play a vital role in diagnosing and monitoring diabetes by measuring blood glucose levels and other relevant biomarkers. In 2024, the diabetes segment is projected to reach a market value of 5,442.27 million USD. The increasing prevalence of diabetes worldwide and the need for effective management strategies drive the demand for diabetes-related IVD kits.
Cardiovascular diseases are a leading cause of mortality and morbidity globally. IVD kits are used to detect biomarkers associated with heart diseases, enabling early diagnosis and intervention. In 2024, the cardiology segment is expected to contribute 4,928.52 million USD to the global IVD Kits market. The growing awareness of cardiovascular health and the need for accurate diagnostic tools support the expansion of this segment.
Cancer is a complex and diverse group of diseases that require precise diagnostic tools for early detection and effective treatment. IVD kits are crucial for identifying cancer biomarkers, monitoring treatment efficacy, and detecting recurrence. In 2024, the oncology segment is projected to generate a revenue of 4,612.87 million USD. The increasing incidence of cancer and advancements in cancer diagnostics drive the demand for oncology-related IVD kits.
Table Global In Vitro Diagnostics IVD Kits Market Size by Application in 2024
Application | Market Size (M USD) 2024 |
Infectious Diseases | 50478.96 |
Diabetes | 5442.27 |
Cardiology | 4928.52 |
Oncology | 4612.87 |
Others | 20124.88 |
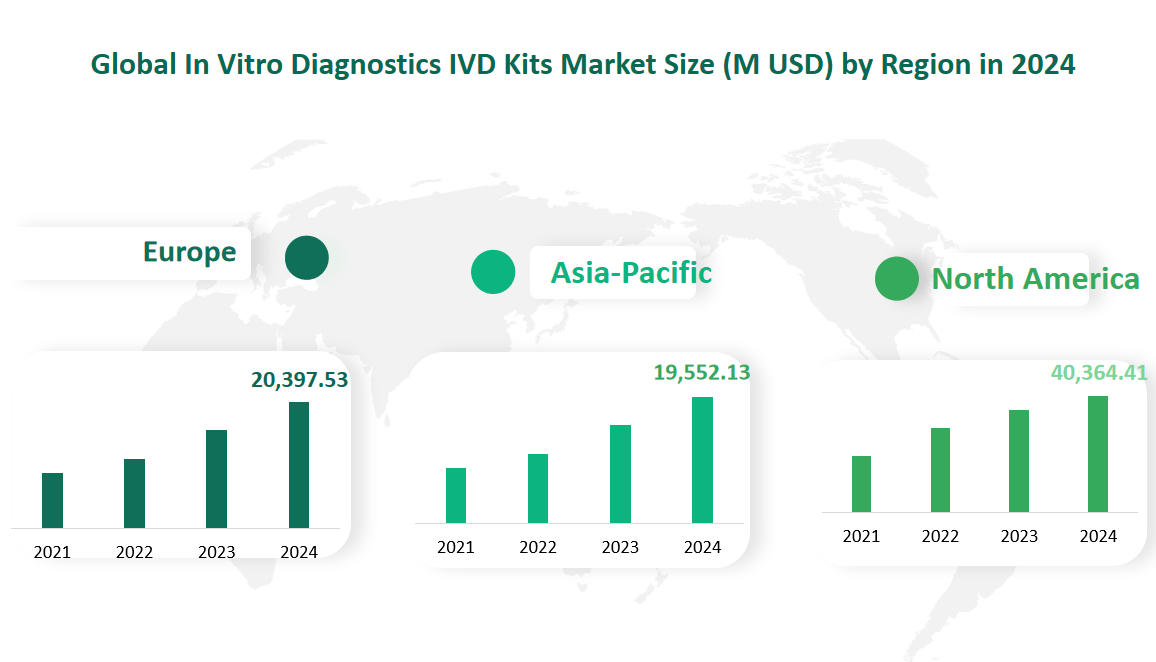
6 Global In Vitro Diagnostics IVD Kits Market Size by Region in 2024
North America is the largest market for IVD Kits, driven by the United States’ advanced healthcare infrastructure and high demand for advanced diagnostic tools. In 2024, the North American market is projected to reach a value of 40,364.41 million USD. The region’s growth is supported by continuous advancements in medical technology, strong regulatory support, and high healthcare spending. The United States, in particular, accounts for the majority of the market share, with Canada contributing significantly as well.
Europe is the second-largest market for IVD Kits, characterized by a strong emphasis on healthcare quality and innovation. In 2024, the European market is expected to reach a value of 20,397.53 million USD. The region’s growth is driven by the presence of several key players, stringent regulatory standards, and a focus on research and development. Germany, France, and the UK are the major contributors to the European market, each with a significant share of the overall revenue.
The Asia-Pacific region is the fastest-growing market for IVD Kits, driven by rapid economic development, increasing healthcare demand, and the growing prevalence of chronic diseases. In 2024, the Asia-Pacific market is projected to reach a value of 19,552.13 million USD. China and Japan are the leading markets in this region, with significant contributions from South Korea, India, and Southeast Asia. The region’s growth is supported by advancements in medical technology, increasing healthcare spending, and the expanding middle-class population.
Figure Global In Vitro Diagnostics IVD Kits Market Size by Region in 2024
7 Major Players in Global In Vitro Diagnostics IVD Kits Market
7.1 Roche Diagnostics
Company Profile
Roche Diagnostics, a division of the Swiss multinational healthcare company Roche Holding AG, is a global leader in the field of in vitro diagnostics. Established in 1896, Roche has a long history of innovation and excellence in healthcare. The company’s headquarters are located in Basel, Switzerland. Roche Diagnostics is renowned for its comprehensive portfolio of diagnostic solutions, including instruments, reagents, and software, which are used in laboratories worldwide to improve patient health and ensure consumer safety.
Business Overview
Roche Diagnostics operates under two main divisions: Pharmaceuticals and Diagnostics. The company’s diagnostic solutions cover a wide range of applications, including infectious diseases, oncology, diabetes, and cardiology. Roche’s commitment to innovation and continuous improvement has positioned it as a leader in the IVD market. The company’s strong presence in both developed and emerging markets ensures that its products are accessible to healthcare providers globally.
Product Overview
Roche Diagnostics offers a broad range of in vitro diagnostic products, including blood tests, molecular diagnostics, and point-of-care testing solutions. The company’s core products include the cobas series of instruments, which are used for PCR-based assays and other molecular diagnostic tests. Roche’s products are designed to provide accurate and reliable results, enabling healthcare providers to make informed decisions about patient care.
Recent Financial Performance
In 2022, Roche Diagnostics reported a revenue of 12,127.25 million USD, with a gross margin of 52.51%.
7.2 Abbott Laboratories
Company Profile
Abbott Laboratories, founded in 1888, is a global healthcare leader with a diverse portfolio of products and services. The company is headquartered in Abbott Park, Illinois, USA. Abbott’s focus on innovation and quality has enabled it to develop a wide range of life-changing technologies that span the spectrum of healthcare, from diagnostics and medical devices to nutritional and branded generic medicines.
Business Overview
Abbott’s diagnostics business is a key driver of the company’s growth. The company offers a comprehensive range of diagnostic solutions, including blood tests, molecular diagnostics, and point-of-care testing. Abbott’s commitment to innovation and quality has positioned it as a leader in the global IVD market. The company’s strong presence in both developed and emerging markets ensures that its products are accessible to healthcare providers worldwide.
Product Overview
Abbott Laboratories offers a wide range of in vitro diagnostic products, including the Alinity series of instruments, which are used for blood tests and molecular diagnostics. The company’s products are designed to provide accurate and reliable results, enabling healthcare providers to make informed decisions about patient care. Abbott’s innovative solutions include rapid diagnostic tests for infectious diseases, diabetes, and cardiology.
Recent Financial Performance
In 2022, Abbott Laboratories reported a revenue of 11,853.13 million USD, with a gross margin of 50.72%.
7.3 Danaher Corporation
Company Profile
Danaher Corporation, founded in 1969, is a global science and technology innovator committed to helping customers solve complex challenges and improve quality of life. The company is headquartered in Washington, D.C., USA. Danaher operates through more than 25 independent companies, each focused on a specific area of science and technology. The company’s diverse portfolio includes products and services in life sciences, diagnostics, water quality, and product identification.
Business Overview
Danaher’s diagnostics business is a key driver of the company’s growth. The company offers a comprehensive range of diagnostic solutions, including molecular diagnostics, point-of-care testing, and clinical laboratory instruments. Danaher’s commitment to innovation and continuous improvement has positioned it as a leader in the global IVD market. The company’s strong presence in both developed and emerging markets ensures that its products are accessible to healthcare providers worldwide.
Product Overview
Danaher Corporation offers a wide range of in vitro diagnostic products, including the BioFire FilmArray system, which is used for rapid molecular diagnostics. The company’s products are designed to provide accurate and reliable results, enabling healthcare providers to make informed decisions about patient care. Danaher’s innovative solutions include rapid diagnostic tests for infectious diseases, oncology, and cardiology.
Recent Financial Performance
In 2022, Danaher Corporation reported a revenue of 7,030.02 million USD, with a gross margin of 46.28%.

