1 Global GMP Plasmid DNA Market Insight Analysis
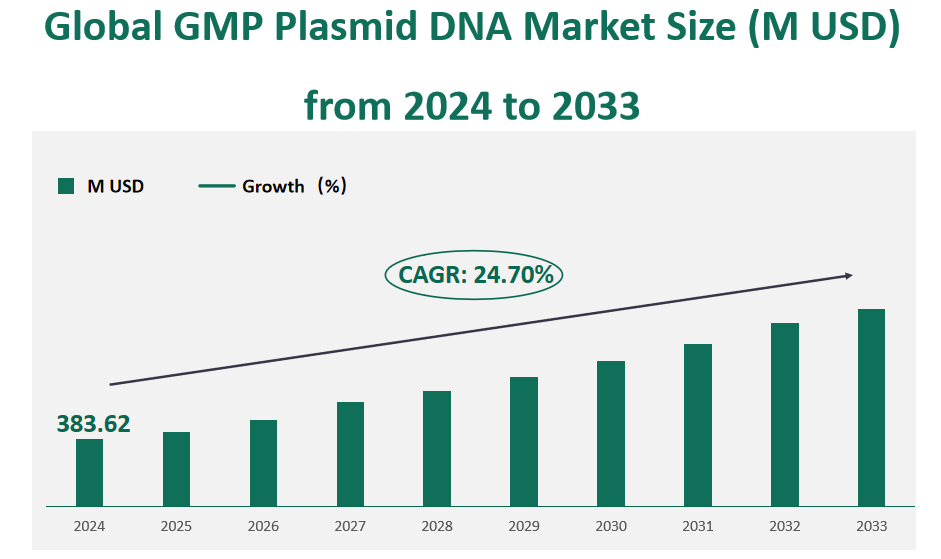
The global GMP Plasmid DNA market size was valued at USD 383.62 million in 2024, with a CAGR of 24.70% from 2024 to 2033.
GMP Plasmid DNA refers to plasmid DNA produced under Good Manufacturing Practice (GMP) conditions. GMP is a set of guidelines and regulations required by agencies that oversee the authorization and licensing of food, beverages, cosmetics, pharmaceutical products, dietary supplements, and medical devices. These guidelines ensure that products are consistently produced and controlled according to high-quality standards. For clinical research applications, especially in gene therapy and genetic vaccination, GMP-grade plasmid DNA is mandatory to ensure safety and efficacy.
Figure Global GMP Plasmid DNA Market Size (M USD) and CAGR (2024-2033)
2 GMP Plasmid DNA Market Growth Drivers and Restraints
Strong downstream demand: Plasmid DNA is widely used in gene therapy, DNA vaccines, immunotherapy and other fields. In gene therapy, it can be used directly as a therapeutic agent; in DNA vaccine development, it can encode specific proteins to stimulate immune responses. As the global demand for these treatments and preventive measures grows, the demand for plasmid DNA has also risen. For example, during the COVID-19 pandemic, the urgent demand for COVID-19 vaccines greatly promoted the production and development of GMP plasmid DNA, and many companies invested in research and development to meet the market demand for vaccines.
Policy support promotes development: Governments’ policy support for the biopharmaceutical industry provides a favorable environment for the GMP plasmid DNA market. Some countries provide financial support and tax incentives for gene therapy-related research and production. For example, the EU clinical research policy provides regulations and convenience for related companies to conduct clinical trials, enabling companies like Cobra Biologics to smoothly produce and develop plasmid DNA, promoting the development of the market.
Technological progress enhances competitiveness: Technological innovation has significantly improved the production efficiency, quality and application effect of plasmid DNA. For example, improvements in production processes have increased the production of plasmid DNA and reduced costs; new vector technologies have improved gene delivery efficiency and expression stability, enhanced its effectiveness in gene therapy and vaccine development, and further expanded market demand.
Price and regulatory challenges: The vector production process required for large-scale clinical trials is expensive and strictly regulated. From raw material selection to final product production, each link must be strictly supervised, which is a huge challenge to the production capacity and equipment conditions of enterprises, increases production costs, and then raises product prices, limiting the further expansion of the market.
Production scale and quality bottlenecks: At present, there are deficiencies in professional technology in terms of scale, complexity and quality assurance of vector production. Although some companies can develop plasmid DNA products, the production scale is small and it is difficult to meet the huge market demand. Moreover, whether the product quality is fully compliant and safe needs to be further confirmed, which also affects the speed of market development.
Gene therapy risks hinder development: Plasmid DNA is mainly used for gene therapy, which has problems such as high cost and mutagenic risk. Viruses and other vectors used in the treatment process may affect non-target cells, and improper gene addition may cause cancer and other problems. These risks limit the promotion of gene therapy and thus have a certain hindering effect on the growth of the GMP plasmid DNA market.
3 Technological Innovations in the GMP Plasmid DNA Market
Production process innovation: Many companies continue to innovate in GMP plasmid DNA production processes. For example, Cobra Bio has continuously improved its DNA platform process since 1998, and now has achieved RNAse free, which can quickly scale up the process, shorten production time, reduce costs, and meet the needs of customers’ gene therapy projects; Aldevron uses proprietary technology to improve plasmid DNA production and provide high-quality products for various research, clinical and diagnostic applications.
Breakthrough in vector technology: Nature Technology Corporation’s Nanoplasmid™ vector performs well and has obvious advantages in indirect and direct applications. Compared with traditional plasmids and other modern alternatives, it is more efficient, safe and economical, does not require cumbersome and expensive post-purification treatment, and meets regulatory requirements. It has been used by more than 100 gene therapy and immunotherapy institutions around the world.
Enhance market competitiveness: Companies integrate resources through mergers and acquisitions to enhance their competitiveness in the market. Kaneka decided to significantly increase the biopharmaceutical production capacity of its European subsidiary and invested in a new large-scale GMP production facility equipped with a 2,200-liter fermenter and related equipment. It is expected to increase production capacity by about four times and expand its business.
Driving changes in the industry landscape: M&A and restructuring have also changed the industry’s competitive landscape. EQT VIII Fund acquired a majority stake in Aldevron. As the world’s leading plasmid DNA supplier, this acquisition may enable Aldevron to further expand its market share with capital support, intensify industry competition, and promote other companies to adjust their strategies to adapt to the new market environment.
Promoting business diversification: LakePharma has become the largest biopharmaceutical CRO in the United States after acquiring Blue Sky BioServices, providing a wider range of protein and antibody-related development services, achieving business diversification, providing customers with one-stop solutions, and enhancing the company’s comprehensive strength in the market. These technological innovations and corporate M&A and restructuring activities are driving the continuous development and change of the GMP plasmid DNA market, affecting the market’s competitive landscape and future trends.
4 Global GMP Plasmid DNA Market Size by Type
Indirect Application refers to the use of plasmid DNA as a raw material or starting material for the production of other therapeutic agents, such as viral vectors, antibodies, or other proteins. This type of plasmid DNA is not directly administered to patients but is used in the production process of these therapeutic agents. The market for Indirect Application has been growing steadily due to the increasing demand for viral vectors and other therapeutic proteins in gene therapy and vaccine development.
In 2024, the revenue for Indirect Application was 209.32 million USD, which is a significant portion of the total market. This growth can be attributed to the increasing number of clinical trials and research projects focusing on gene therapy and vaccine development. The use of plasmid DNA in these applications is crucial, as it provides a reliable and efficient method for producing the necessary therapeutic agents.
Direct Application involves the use of plasmid DNA as an active pharmaceutical ingredient (API) that is directly injected into patients. This type of application is primarily used in gene therapy, where the plasmid DNA is designed to deliver specific genetic information to the patient’s cells. The market for Direct Application has also been growing, driven by advancements in gene therapy technologies and the increasing number of clinical trials exploring the potential of DNA-based therapies.
In 2024, the revenue for Direct Application was 174.30 million USD, representing a substantial portion of the total market. The growth in this segment is driven by the increasing acceptance and success of gene therapy treatments, which rely heavily on the use of high-quality GMP plasmid DNA. As more gene therapy products move from research to clinical trials and eventually to market approval, the demand for Direct Application plasmid DNA is expected to continue to rise.
Table Global GMP Plasmid DNA Market Size and Share by Type in 2024
Type | Market Size (M USD) 2024 | Market Share 2024 |
|---|---|---|
Indirect Application | 209.32 | 54.57% |
Direct Application | 174.30 | 45.43% |
5 Global GMP Plasmid DNA Market Size by Application
DNA Vaccines: In 2024, the revenue of the DNA Vaccines segment is forecasted to reach approximately 138.84 million USD. It holds a market share of about 36.19%. DNA vaccines work by injecting DNA that codes for specific proteins from a pathogen into the body. The cells then synthesize these proteins based on the genetic code, triggering an immune response. The continuous research and development in vaccine technology, especially in response to emerging infectious diseases, contribute to the growth of this segment. For example, during the COVID – 19 pandemic, the development of DNA – based vaccines has received a significant boost, leading to an increased demand for GMP Plasmid DNA in this application.
Gene Therapy: The Gene Therapy segment is expected to generate a revenue of around 130.47 million USD in 2024, with a market share of 34.01%. Gene therapy involves using plasmids to transfer genes to cells in order to treat various diseases. This can be achieved by improving the resistance of cells to diseases or enhancing their growth rates. The growing understanding of genetic diseases and the development of advanced gene – transfer techniques have led to an increased use of GMP Plasmid DNA in gene therapy. However, the high cost and potential risks associated with gene therapy still pose challenges to its widespread adoption.
Immunotherapy: Immunotherapy is projected to have a revenue of about 88.76 million USD in 2024, accounting for a market share of 23.14%. This type of cancer treatment uses the body’s immune system to fight cancer. GMP Plasmid DNA plays a crucial role in the development of immunotherapy drugs. As the research in immunotherapy progresses and more effective treatment methods are developed, the demand for GMP Plasmid DNA in this segment is likely to increase.
Table Global GMP Plasmid DNA Market Size and Share by Application in 2024
Application | Market Size (M USD) 2024 | Market Share 2024 |
|---|---|---|
DNA Vaccines | 138.84 | 36.19% |
Gene Therapy | 130.47 | 34.01% |
Immunotherapy | 88.76 | 23.14% |
Others | 25.54 | 6.66% |
6 Global GMP Plasmid DNA Market Size by Region
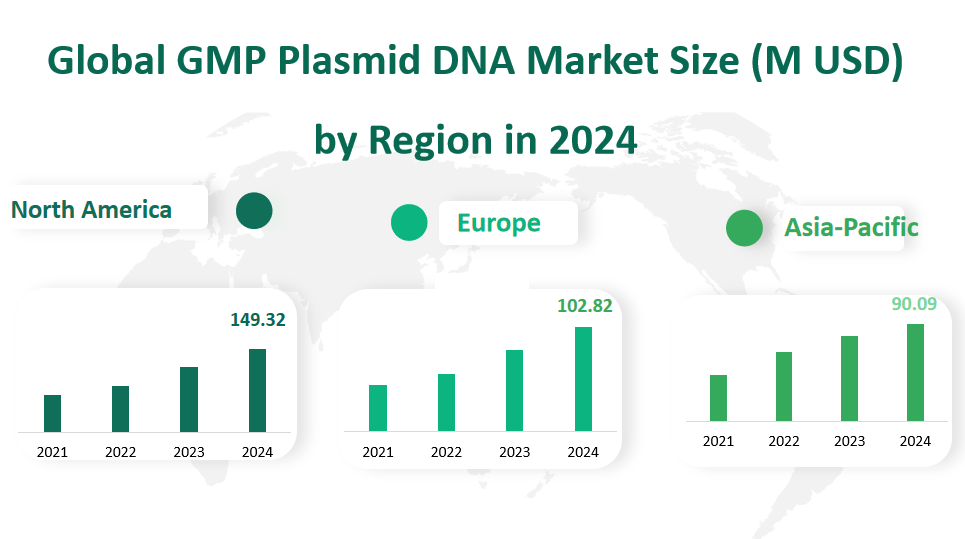
North America: North America is expected to be the largest market in 2024, with a revenue of approximately 149.32 million USD and a market share of 38.92%. The region has a well – developed biopharmaceutical industry, with a large number of research institutions and pharmaceutical companies. For example, companies like Aldevron, which is based in North America, are major players in the global GMP Plasmid DNA market. The high level of investment in research and development, along with strong government support for biotech innovation, drives the growth of the market in this region.
Europe: Europe is projected to have a revenue of around 102.82 million USD in 2024, with a market share of 26.80%. Countries like Germany, the UK, and France have a long – standing history in biopharmaceutical research and production. Richter – Helm Biologics in Germany is a key player in the European market. The region’s strict regulatory environment ensures high – quality production of GMP Plasmid DNA, but it also poses challenges for new entrants.
Asia – Pacific: The Asia – Pacific region is experiencing rapid growth in the GMP Plasmid DNA market. In 2024, its revenue is expected to reach about 90.09 million USD, with a market share of 23.48%. China, in particular, has been a major contributor to the growth in this region. The Chinese government’s increasing investment in biotech research, along with a large population that provides a vast market, has led to a significant expansion of the GMP Plasmid DNA market. Additionally, countries like India and South Korea are also emerging as important players in the region.
Latin America: Latin America is expected to have a revenue of around 28.61 million USD in 2024, with a market share of 7.46%. Brazil and Mexico are the major markets in this region. The growth in Latin America is driven by the increasing investment in healthcare and biotech research. However, the region still faces challenges such as a lack of advanced technology and infrastructure compared to other regions.
Middle East & Africa: The Middle East & Africa region is forecasted to have a revenue of approximately 12.78 million USD in 2024, with a market share of 3.33%. Although the market size is relatively small compared to other regions, countries like Turkey, Saudi Arabia, and the UAE are showing increasing interest in biopharmaceutical development. The growth in this region is mainly driven by government initiatives to improve healthcare services and invest in advanced medical technologies.
Figure Global GMP Plasmid DNA Market Size (M USD) by Region in 2024
7 Global GMP Plasmid DNA Market Analysis by Major Players
Eurogentec
Company Profile
Eurogentec is a leading supplier of high-quality GMP plasmid DNA for various applications, including gene therapy and vaccine development. Established in 1985, Eurogentec has a strong presence in the European market and has expanded its operations globally over the years. The company is headquartered in the United States and operates manufacturing facilities in Europe, ensuring compliance with stringent regulatory standards.
Business Overview
Eurogentec offers a wide range of products and services tailored to the needs of the life science, biotechnology, pharmaceutical, and diagnostic markets. The company specializes in the production of reagents, kits, specialty products, and custom services. Eurogentec’s expertise in GMP plasmid DNA production is particularly notable, as it provides critical materials for the development of viral vectors and therapeutic proteins.
Product Offered
Eurogentec’s GMP plasmid DNA products are designed to meet the highest quality standards, ensuring safety and efficacy in clinical applications. The company offers plasmid DNA for both indirect and direct applications. For indirect applications, Eurogentec provides plasmid DNA used as a starting material for the production of viral vectors (e.g., lentivirus and AAV) and in vitro transcribed RNA (e.g., mRNA). For direct applications, Eurogentec produces plasmid DNA APIs for use in DNA vaccines and therapeutic non-viral plasmid DNA-mediated gene therapy applications. Their products are widely recognized for their high transfection efficiency, effective gene delivery, and stable gene expression.
Aldevron
Company Profile
Aldevron, founded in 1998, is a global leader in the production of GMP plasmid DNA. The company has a strong reputation for its high-quality products and innovative manufacturing processes. Aldevron operates manufacturing facilities in North America and Europe, ensuring a global reach and compliance with international regulatory standards. The company’s headquarters are located in Fargo, North Dakota, USA.
Business Overview
Aldevron specializes in the development and manufacturing of plasmid DNA, proteins, enzymes, antibodies, and other biologicals. The company’s products are used in a wide range of applications, including gene therapy, vaccine development, and diagnostic research. Aldevron’s custom development and manufacturing capabilities enable scientists worldwide to develop groundbreaking new therapies with a focus on quality, speed, and innovation.
Product Offered
Aldevron offers a variety of in-stock plasmid DNA items for research use in mammalian cells. The company has perfected plasmid DNA production over more than 20 years, using proprietary technology to manufacture DNA for a wide range of research, preclinical, clinical, and diagnostic applications. Aldevron’s GMP plasmid DNA products are designed to meet the stringent requirements of clinical trials and regulatory agencies. Their plasmid DNA is used in both indirect applications (e.g., production of viral vectors and therapeutic proteins) and direct applications (e.g., DNA vaccines and gene therapy).
Richter-Helm Biologics
Company Profile
Richter-Helm Biologics, established in 1901, is a well-established biotech company with a global presence. The company is headquartered in Germany and operates manufacturing facilities that comply with the highest international standards. Richter-Helm Biologics has a strong reputation for its high-quality products and reliable manufacturing processes.
Business Overview
Richter-Helm Biologics specializes in the production of recombinant proteins and peptides, plasmid DNA, and bacterial vaccines. The company’s products are used in various applications, including gene therapy, vaccine development, and diagnostic research. Richter-Helm Biologics has a long history of providing high-quality products and services to the biotechnology and pharmaceutical industries.
Product Offered
Richter-Helm Biologics’ GMP plasmid DNA products are designed to meet the stringent requirements of clinical trials and regulatory agencies. The company’s production facilities have received production licenses from local German authorities and have been certified by the European Medicines Agency (EMA), the U.S. Food and Drug Administration (FDA), and other international bodies. Richter-Helm Biologics’ plasmid DNA is used in both indirect applications (e.g., production of viral vectors and therapeutic proteins) and direct applications (e.g., DNA vaccines and gene therapy). The company’s products are known for their high quality, reliability, and compliance with international standards.

