1 Global Fluticasone Propionate Inhalers Market Insight Analysis
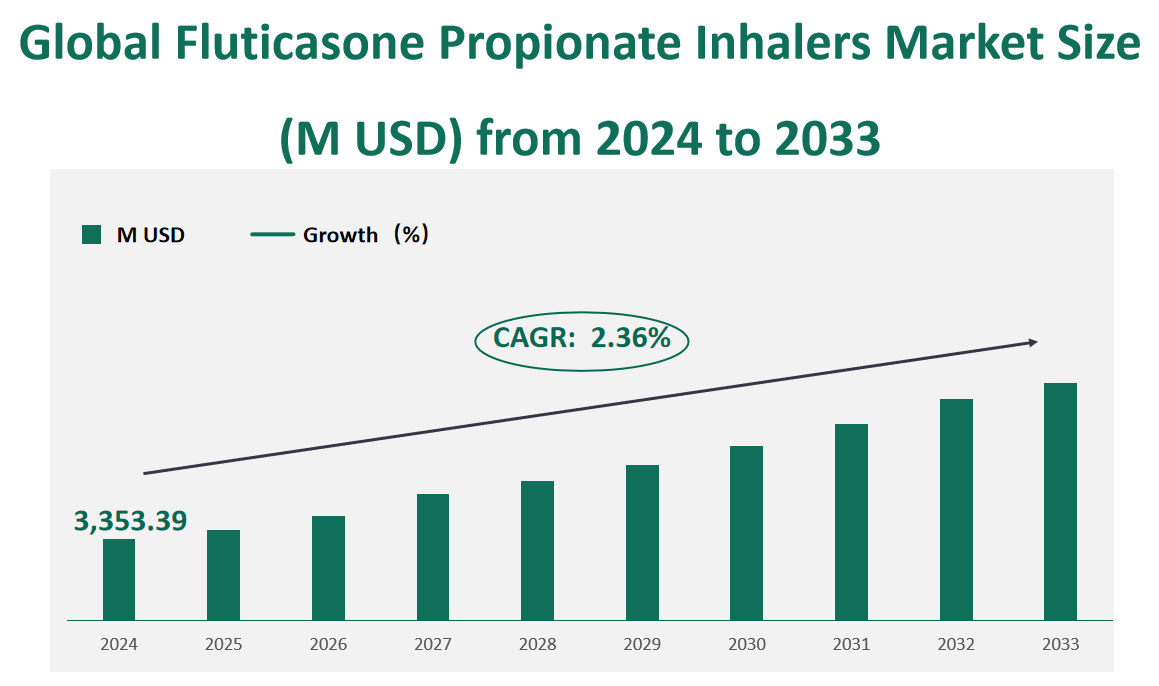
The global fluticasone propionate inhalers market is valued at USD 3,353.39 million in 2024, with a CAGR of 2.36% from 2024 to 2033.
Fluticasone Propionate Inhalers are medical devices that deliver corticosteroids directly to the lungs to reduce inflammation and manage symptoms of asthma and other chronic respiratory diseases. These inhalers are crucial for patients who require long-term management of their conditions. The market for these inhalers is defined by the demand from healthcare providers, pharmacies, and patients, as well as the supply from pharmaceutical companies and distributors.
Figure Global Fluticasone Propionate Inhalers Market Size (M USD) and CAGR (2024-2033)
2 Fluticasone Propionate Inhalers Market Growth Drivers and Restraints
From the perspective of driving factors, the increase in market penetration and the enhancement of health awareness play a key role. The cause of asthma is unknown and is affected by genetic and environmental factors, and the patient population is huge. As consumers’ health awareness increases, they pay more attention to disease prevention and treatment, and their healthcare expenditures increase, and their willingness to buy fluticasone inhalers continues to rise. Globally, more and more people are aware of the importance of standardized treatment of asthma and are actively seeking effective treatment drugs, which has promoted the increase in market penetration of this product and provided a solid demand foundation for market growth.
The popularity of self-treatment is also an important driving force for market growth. Consumers’ demand for self-use drugs continues to grow, and they tend to choose over-the-counter (OTC) drugs for self-treatment. A large number of adults and parents in the United States give priority to using OTC drugs before seeking medical treatment. This trend has led to the continuous expansion of the fluticasone inhaler market. At the same time, the diversified development of sales channels, such as the increase in pharmaceutical companies, drugstores, supermarkets, retail stores and hospital pharmacies, as well as the rise of the Internet and mobile networks, have made it more convenient for consumers to purchase this product, further stimulating market demand.
However, market development also faces many limiting factors. Brand barriers and policy pressure are one of the main obstacles. Building a good brand reputation requires time accumulation and a lot of advertising investment. With the increase in media advertising and promotion costs, the cost of building new product brands has increased dramatically, and new entrants face high brand entry barriers. In addition, the policy pressure on the pharmaceutical industry has gradually increased, drug advertising supervision has become stricter, and drug price bidding has compressed the profit margins of enterprises, further increasing the difficulty of building new brands and limiting the further expansion of the market and the entry of new enterprises.
Product side effects also have a certain impact on market development. Fluticasone inhalers have side effects such as white spots in the mouth/tongue, signs of infection, and vision problems. Although the benefits outweigh the disadvantages when used under the guidance of a doctor, these side effects may still cause some patients to have concerns about the product, affecting their willingness to buy, and to a certain extent hindering the growth of the market.
3 Technological Innovations in the Fluticasone Propionate Inhalers Market
Technological innovation has injected strong impetus into market development. With the improvement of global scientific and technological levels, the production technology of fluticasone inhalers has been continuously innovated. In the field of drug research and development, artificial intelligence has played an important role. Some companies use artificial intelligence algorithms to quickly screen out effective drugs for specific diseases, greatly shortening the research and development cycle and reducing research and development costs. Take Atomwise as an example. Its algorithm can predict effective drugs in a short time when studying drugs for the treatment of Ebola virus infection. This efficient research and development model provides new ideas and methods for the innovative research and development of drugs such as fluticasone inhalers.
In terms of supply chain, technological innovation has also brought changes. The application of artificial intelligence and robotics technology not only shortens the drug design time, but also optimizes the production process and improves production efficiency. Robots can automate multiple tasks during the manufacturing process. For example, robots provided by Denso Robotics can assist in heavy production work, reduce labor costs, improve product quality and production efficiency, and ensure a more stable and efficient supply of fluticasone inhalers.
4 Global Fluticasone Propionate Inhalers Market Size by Type
For the 60 Metered Sprays type, the market revenue reached 859.77 million US dollars, accounting for 25.64% of the total market value. The consistent demand for 60 Metered Sprays may be due to its suitability for certain patient groups, such as those with milder symptoms or for short – term use. Its compact size and lower dosage per spray might also make it more convenient for some users, especially those who require a less intense medication delivery.
The 120 Metered Sprays type dominated the market in 2024. It generated a market revenue of 2188.86 million US dollars, holding a significant 65.27% of the total market share. This type has been the most popular among consumers and healthcare providers. The higher number of metered sprays in this type likely provides a more extended treatment period without the need for frequent replacement, which is cost – effective and convenient for patients with chronic conditions. Moreover, the dosage delivery in 120 Metered Sprays may be optimized to meet the needs of a large portion of patients suffering from asthma or other respiratory diseases.
The 150 Metered Sprays type had a market revenue of 304.75 million US dollars in 2024, corresponding to a market share of 9.09%. Although it has a relatively smaller share compared to the other two types, it still serves a specific segment of the market. This type might be preferred by patients who require a higher cumulative dosage over an extended period or for those with more severe conditions. The larger number of metered sprays can potentially reduce the frequency of purchasing new inhalers, which is beneficial for long – term treatment.
Table Global Fluticasone Propionate Inhalers Market Size and Share by Type in 2024
Type | Market Size (M USD) 2024 | Market Share 2024 |
|---|---|---|
60 Metered Sprays | 859.77 | 25.64% |
120 Metered Sprays | 2188.86 | 65.27% |
150 Metered Sprays | 304.75 | 9.09% |
5 Global Fluticasone Propionate Inhalers Market Size by Application
For the application of fluticasone propionate inhalers for kids, the market revenue in 2024 was 507.37 million US dollars. This accounted for 15.13% of the total market value. Although this segment has a relatively smaller share compared to the adult – oriented application, it still represents a significant market size. The demand for fluticasone propionate inhalers for kids is driven by the increasing prevalence of respiratory diseases among children, such as asthma. As parents and healthcare providers become more aware of the importance of proper treatment for childhood respiratory conditions, the market for pediatric inhalers is expected to continue to grow steadily.
On the other hand, the application for adults dominated the global fluticasone propionate inhalers market in 2024. The market revenue for adult – oriented inhalers was 2846.02 million US dollars, which constituted 84.87% of the total market value. The large share of the adult segment can be attributed to several factors. Firstly, the adult population is generally larger, and the incidence of respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD) is relatively high among adults. Additionally, the aging population in many parts of the world has led to an increased demand for medications to manage respiratory conditions.
Table Global Fluticasone Propionate Inhalers Market Size and Share by Application in 2024
Application | Market Size (M USD) 2024 | Market Share 2024 |
|---|---|---|
Kids | 507.37 | 15.13% |
Adults | 2846.02 | 84.87% |
6 Global Fluticasone Propionate Inhalers Market Size by Region
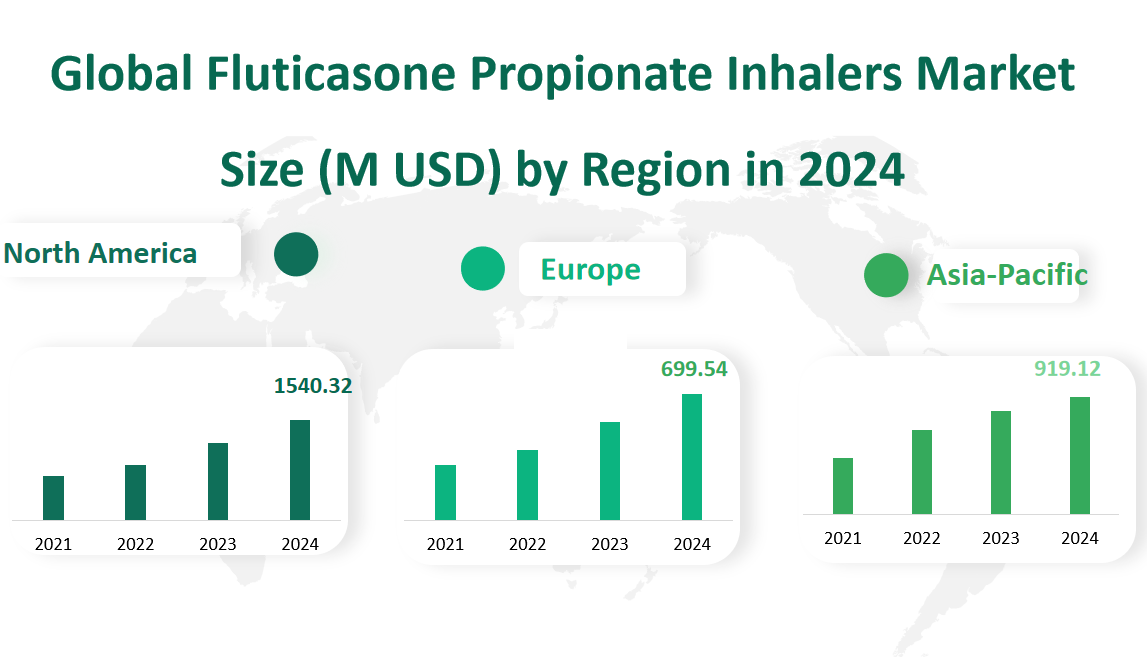
North America has consistently been a dominant region in the global Fluticasone Propionate Inhalers market. In 2024, the market value is projected to reach approximately $1540.32 million. This region’s significant market share can be attributed to its advanced healthcare infrastructure, high healthcare spending, and the presence of key pharmaceutical companies. The region’s ability to quickly adopt new treatments and the presence of a large patient base with respiratory conditions contribute to its leading position.
Europe follows closely behind North America in terms of market size. For 2024, the projected market value is around $699.54 million. The European market is characterized by its stringent regulatory environment and the presence of several generic drug manufacturers. The region’s healthcare systems, which often provide extensive coverage for respiratory treatments, also play a crucial role in driving demand for Fluticasone Propionate Inhalers.
The Asia Pacific region is expected to show robust growth in the coming years. In 2024, the market value is projected to be about $919.12 million. This region’s growth is driven by the increasing prevalence of respiratory diseases, growing awareness about asthma and other conditions, and improving healthcare access. Countries like China and Japan, with their large populations and aging demographics, are expected to contribute significantly to the region’s market growth.
South America, while smaller compared to North America and Europe, is still a significant player in the global market. The projected market value for 2024 is approximately $155.14 million. The region’s growth is influenced by factors such as increasing healthcare investment and the rising middle class, which is leading to better access to healthcare and a growing demand for effective respiratory treatments.
The Middle East and Africa region, though currently the smallest in terms of market value, is expected to see growth due to factors like population growth and increasing awareness about respiratory health. In 2024, the market value is projected to be around $39.27 million. The region’s potential is significant, especially as improvements in healthcare infrastructure and economic development could lead to increased demand for Fluticasone Propionate Inhalers.
Figure Global Fluticasone Propionate Inhalers Market Size (M USD) by Region in 2024
7 Global Fluticasone Propionate Inhalers Market Analysis by Major Players
GSK
Company Profile: GSK, headquartered in the UK, is a renowned pharmaceutical company with a global presence. Established in 2000, GSK has established itself as a leader in the pharmaceutical industry through its commitment to research and development.
Business Overview: GSK operates worldwide, focusing on the development, manufacturing, and marketing of vaccines, prescription, and over-the-counter medicines, as well as health-related consumer products. The company provides solutions for a range of conditions including infections, depression, skin conditions, asthma, heart and circulatory diseases, and cancer.
Product Offered: GSK’s product portfolio includes FLOVENT HFA, which is used to treat asthma in patients aged four years and older. FLOVENT HFA contains fluticasone propionate, a synthetic corticosteroid that helps reduce airway inflammation. This product is recommended by health organizations like the National Institutes of Health for people with persistent asthma.
Mylan
Company Profile: Mylan, established in 1961 and headquartered in the Netherlands, is a global generic and specialty pharmaceuticals company. The company has a significant global presence and is known for its extensive range of pharmaceutical products.
Business Overview: Mylan operates an active pharmaceutical ingredient manufacturer and runs a specialty business focused on respiratory, allergy, and psychiatric therapies. The company launched its Fluticasone Propionate Inhalers in 2019, marking a significant expansion in its respiratory product line.
Product Offered: Mylan’s product, Wixela Inhub®, is the first FDA-approved generic version of ADVAIR DISKUS®. It contains the same active ingredients and has been demonstrated in clinical studies to help improve lung function, making it a suitable choice for asthma and COPD patients. Wixela Inhub® is a result of a rigorous 10-year research and development program, ensuring its effectiveness and safety.
Teva
Company Profile: Teva, founded in 1901 and headquartered in Israel, operates as a pharmaceutical company with a global reach. The company has a rich history and is known for its generic and branded human pharmaceuticals.
Business Overview: Teva develops, manufactures, and markets generic and branded human pharmaceuticals, as well as active pharmaceutical ingredients. The company’s products are used to treat a wide range of conditions, contributing significantly to global healthcare.
Product Offered: Teva offers a generic version of AirDuo RespiClick® Inhalation Powder, which is a respiratory tract agent. This product is an authorized generic, indicating its equivalence to the branded product in terms of safety and efficacy. It is available in a size of 0.45 g/60 actuations, providing a convenient and effective treatment option for respiratory conditions.

