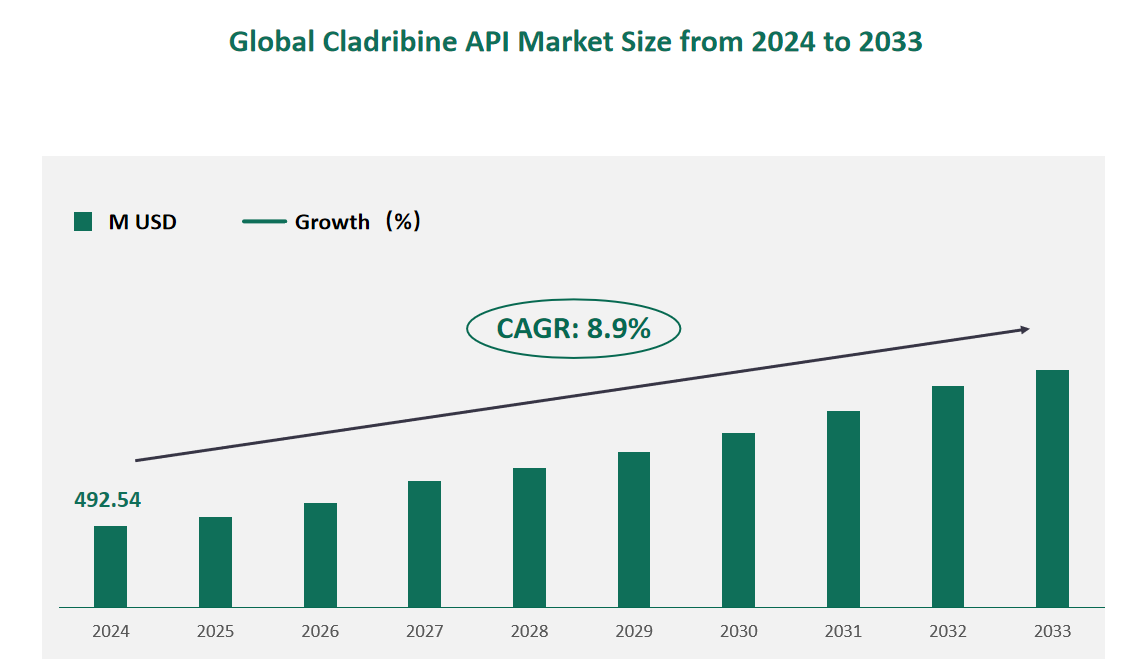
1 Global Cladribine API Market Size (Value) and CAGR (2024-2033)
In 2024, the global Cladribine API market was valued at USD 492.54 million, with a CAGR of 8.9% from 2024 to 2033.
API is also called active pharmaceutical ingredient; API refers to the raw material drug used in the production of various preparations and is the active ingredient in the preparation. Cladribine inhibits DNA synthesis and repair; cladribine is used to treat hairy cell leukemia and multiple sclerosis (MS).
Figure Global Cladribine API Market Size (M USD) and CAGR 2024-2033
2 Cladribine API Market Drivers
One of the primary drivers is the continuous development of the pharmaceutical industry. The pharmaceutical sector is a cornerstone of medical progress, constantly evolving through advancements in technology and globalization. Cladribine API, originally developed in the 1970s for treating various blood cancers, has been repurposed for treating multiple sclerosis (MS) due to its ability to reduce T and B lymphocytes. As the pharmaceutical industry grows, so does the demand for Cladribine API, which is essential for producing these life-saving medications.
In the digital age, e-commerce platforms have revolutionized the way products are sold and distributed. For the Cladribine API industry, this means broader market reach and more efficient sales channels. The growth of cross-border e-commerce, supported by advanced technology and favorable policies, has further expanded the global market for Cladribine API. This trend not only increases sales volumes but also enhances the industry’s ability to meet the growing global demand for pharmaceuticals.
Government policies also play a crucial role in driving the Cladribine API market. For instance, China has implemented policies that reduce tariffs on imported drugs and raw materials, making it more cost-effective to produce and distribute Cladribine API. These policies not only benefit domestic manufacturers but also attract international players to invest in the market.
3 Cladribine API Market Restraints
While the Cladribine API market enjoys significant growth drivers, it also faces several development constraints that can hinder its expansion. One of the most critical constraints is the stringent quality control requirements. The pharmaceutical industry is highly regulated, with quality control being paramount due to its direct impact on human health. Cladribine API manufacturers must adhere to strict standards set by regulatory bodies worldwide. Any deviation in quality can lead to severe consequences, including recalls, legal actions, and loss of market trust. Ensuring consistent quality across the production process, from raw materials to final products, is a significant challenge for manufacturers.
Another constraint is the high cost of production and research and development (R&D). The Cladribine API industry requires substantial investment in advanced technologies and R&D to maintain product quality and efficacy. The cost of compliance with regulatory standards, coupled with the need for continuous innovation, places a financial burden on manufacturers. This high cost can limit the entry of new players into the market and slow down the overall growth of the industry.
4 Global Cladribine API Market Size and Share by Type in 2024
Direct Distribution refers to the process where manufacturers deliver products directly to downstream customers without involving intermediaries such as wholesalers or distributors. This channel allows manufacturers to maintain control over the sales process, ensuring direct communication with customers and potentially higher profit margins. In 2024, the direct distribution channel is expected to account for $425.45 million of the total market value, representing approximately 86.38% of the total Cladribine API market. The growth of this channel is driven by the increasing adoption of e-commerce platforms by manufacturers. These platforms enable direct sales to customers, reducing the need for intermediaries and enhancing customer relationships.
Indirect Distribution involves the use of intermediaries such as wholesalers, distributors, and agents to sell the product. This channel is particularly useful for expanding market reach and accessing a broader customer base. It leverages the established networks and logistics capabilities of intermediaries to facilitate sales. In 2024, the indirect distribution channel is projected to contribute $67.08 million to the total market value, representing about 13.62% of the total Cladribine API market. The growth of the indirect distribution channel is influenced by the expanding global market and the need for manufacturers to reach customers in diverse regions. Intermediaries often have specialized knowledge and established relationships in specific markets, which can enhance market penetration and sales efficiency.
Table Global Cladribine API Market Size and Share by Type in 2024
Type | Market Size (M USD) 2024 | Market Share 2024 |
Direct Distribution | 425.45 | 86.38% |
Indirect Distribution | 67.08 | 13.62% |
5 Global Cladribine API Market Consumption (g) by Application in 2024
Leukemia is a type of cancer that affects the blood and bone marrow. Cladribine API is used to treat various forms of leukemia, particularly hairy cell leukemia. In 2024, the consumption of Cladribine API for leukemia treatment is expected to reach 1,034 grams. This represents a significant portion of the market, driven by the increasing prevalence of leukemia and the need for effective treatments. The growth in this segment is further supported by advancements in medical research and the continuous development of new therapeutic applications for Cladribine.
Multiple sclerosis is a chronic disease that affects the central nervous system. Cladribine API has been developed as an oral formulation for the treatment of MS, offering a convenient and effective option for patients. In 2024, the consumption of Cladribine API for MS treatment is projected to be 15,735 grams, accounting for the majority of the market. The high consumption in this segment is attributed to the increasing incidence of MS and the growing demand for oral treatments that improve patient compliance and quality of life. Additionally, the market benefits from ongoing clinical trials and regulatory approvals that expand the use of Cladribine API in MS treatment.
Table Global Cladribine API Market Consumption (g) by Application in 2024
Application | Market Consumption (g) 2024 | Market Share 2024 |
Multiple Sclerosis (MS) | 15735 | 91.48% |
Leukemia | 1034 | 6.01% |
Others | 432 | 2.51% |
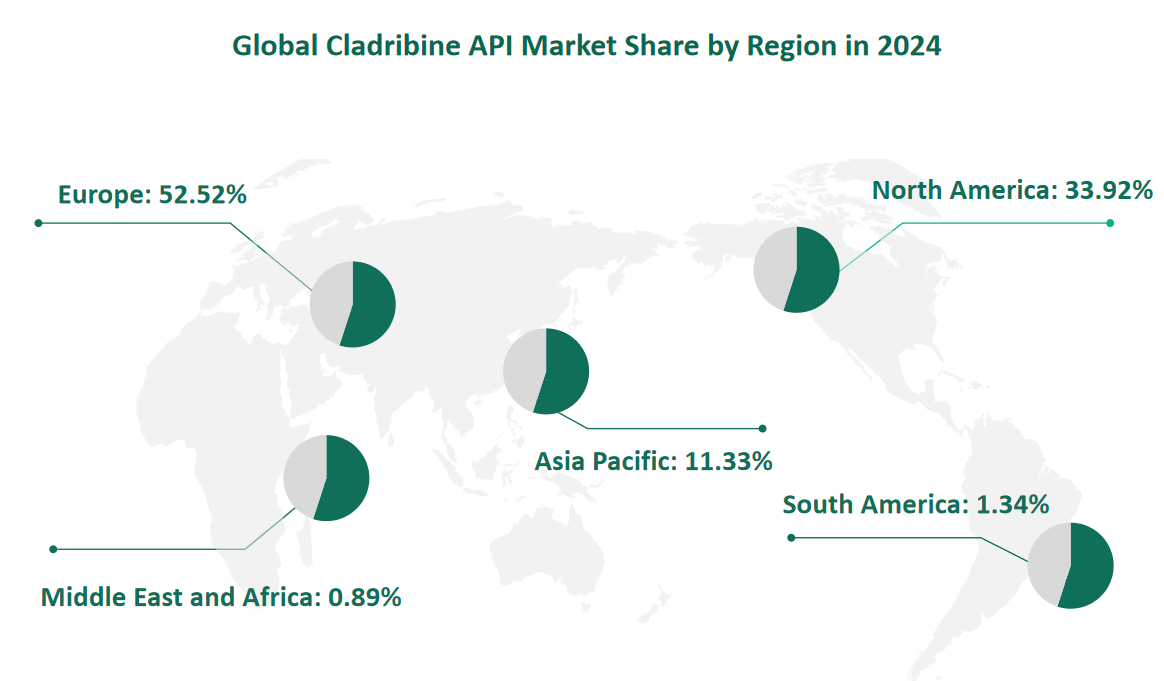
6 Global Cladribine API Market Size by Region in 2024
North America, comprising the United States and Canada, is a significant market for Cladribine API. In 2024, the market value in North America is projected to be $167.06 million, representing approximately 33.92% of the global market. The growth in this region is driven by the high demand for advanced pharmaceuticals, robust healthcare infrastructure, and significant investments in medical research. The United States, in particular, is a major consumer of Cladribine API due to its large pharmaceutical market and the prevalence of diseases such as leukemia and MS.
Europe is another major market for Cladribine API, with a projected market value of $258.69 million in 2024, accounting for 52.52% of the global market. The growth in Europe is influenced by the region’s strong pharmaceutical industry, advanced healthcare systems, and high demand for effective treatments. Countries such as Germany, the UK, and France are key markets within Europe, contributing significantly to the overall consumption of Cladribine API. The region also benefits from favorable regulatory policies that support the development and distribution of pharmaceuticals.
The Asia Pacific region is a rapidly growing market for Cladribine API, with a projected market value of $55.79 million in 2024, representing 11.33% of the global market. The growth in this region is driven by the increasing prevalence of diseases, improving healthcare infrastructure, and growing demand for advanced pharmaceuticals. China and India are key markets within the Asia Pacific region, contributing significantly to the overall consumption. The region also benefits from cost advantages in production, making it an attractive destination for Cladribine API manufacturing.
Figure Global Cladribine API Market Share by Region in 2024
7 Major Players in Global Cladribine API Market
7.1 Zhejiang Hisun Pharmaceutical Co. Ltd.
Company Profile:
Zhejiang Hisun Pharmaceutical Co. Ltd. is a comprehensive pharmaceutical company that integrates multi-regional research, production, sales, raw materials, and preparations. Established in 1956 and headquartered in China, Zhejiang Hisun has grown to become one of China’s largest manufacturers of antibiotics and anti-tumor drugs. The company is recognized as one of the top 500 Chinese manufacturing enterprises and among the top 100 Chinese pharmaceutical companies. Zhejiang Hisun focuses on the R&D, production, and sales of innovative drugs, biopharmaceuticals, generic drugs, and high-end active drug components (API).
Business Overview:
Zhejiang Hisun Pharmaceutical Co. Ltd. operates globally, with a strong presence in the pharmaceutical industry. The company’s product portfolio covers more than ten treatment areas, including anti-tumor, anti-infection, cardiovascular, endocrine, immunosuppressive, anti-depression, and orthopedic treatments. Zhejiang Hisun’s commitment to innovation and quality has positioned it as a leading player in the Cladribine API market.
Product and Service Analysis:
Zhejiang Hisun offers Cladribine API with specifications approved by Chinese regulatory authorities. The company’s Cladribine API is known for its high purity and compliance with international standards. Zhejiang Hisun’s robust manufacturing capabilities and stringent quality control processes ensure the reliability and efficacy of its products.
Recent Financial Performance:
In the most recent year, Zhejiang Hisun Pharmaceutical Co. Ltd. reported a revenue of $136.43 million from Cladribine API sales.
7.2 ScinoPharm Taiwan, Ltd.
Company Profile:
ScinoPharm Taiwan, Ltd. is a global active pharmaceutical ingredient (API) manufacturer, established in 1997 and headquartered in Taiwan. The company is renowned for its advanced cGMP production facilities, which have passed quality inspections by multiple international regulatory bodies, including the Taiwan FDA (TFDA), EU EMA, EDQM, US FDA, Australian TGA, Japanese PMDA, Korean FDA, and German authorities.
Business Overview:
ScinoPharm Taiwan, Ltd. offers a wide range of API and intermediate development and manufacturing services. The company supplies APIs to world-renowned pharmaceutical companies and generic drug manufacturers, as well as providing outsourcing services for new drug research and development companies and patented pharmaceutical companies. ScinoPharm’s comprehensive research and development capabilities and robust manufacturing infrastructure position it as a key player in the global Cladribine API market.
Product and Service Analysis:
ScinoPharm’s Cladribine API is produced with advanced cGMP facilities, ensuring high quality and compliance with international standards. The company’s Cladribine API is used in the treatment of various cancers, particularly leukemia, and has gained a reputation for its reliability and efficacy. ScinoPharm’s commitment to continuous improvement and innovation ensures that its products meet the evolving needs of the pharmaceutical industry.
Recent Financial Performance:
In the most recent year, ScinoPharm Taiwan, Ltd. reported a revenue of $111.24 million from Cladribine API sales.
7.3 Reliable Biopharmaceutical LLC
Company Profile:
Reliable Biopharmaceutical LLC, established in 1968 and headquartered in the USA, is an American manufacturer of high-quality active pharmaceutical ingredients and high-purity ingredients for human care. The company is known for its integrated API development and cGMP manufacturing capabilities, which ensure safe and expedited market approval.
Business Overview:
Reliable Biopharmaceutical LLC operates primarily in North America, with a focus on providing safe and high-quality APIs. The company’s comprehensive project management, including complex science, regulatory, and analytical expertise, ensures that its products meet the highest standards of quality and compliance. Reliable Biopharmaceutical’s commitment to innovation and quality control has positioned it as a leading player in the Cladribine API market.
Product and Service Analysis:
Reliable Biopharmaceutical LLC offers Cladribine API with specifications that meet international standards. The company’s robust manufacturing processes and stringent quality control measures ensure the reliability and efficacy of its products. Reliable Biopharmaceutical’s commitment to continuous improvement and innovation ensures that its products meet the evolving needs of the pharmaceutical industry.
Recent Financial Performance:
In the most recent year, Reliable Biopharmaceutical LLC reported a revenue of $68.42 million from Cladribine API sales.

