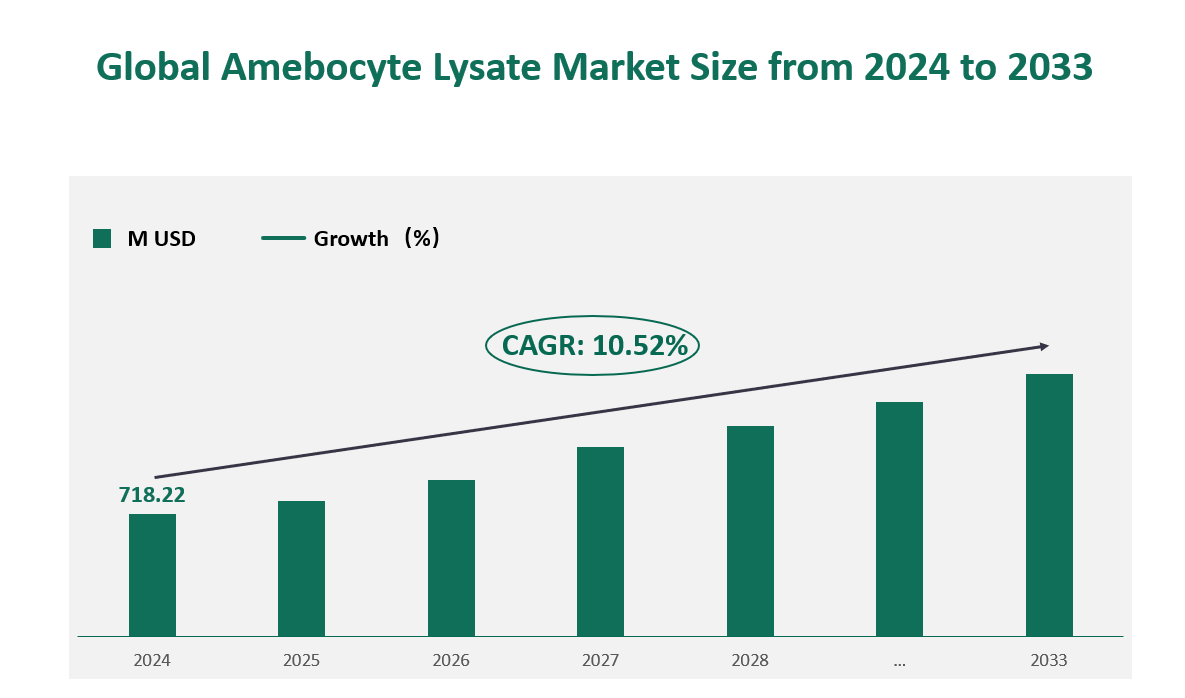
1 Global Amebocyte Lysate Market Size (Revenue) and CAGR (2024-2033)
Global Amebocyte Lysate market generated revenue of USD 718.22 Million in 2024 with a CAGR of 10.52% during 2024 to 2033.
The Amebocyte Lysate market is currently experiencing robust growth, driven by several key factors. One of the primary drivers is the increasing demand for endotoxin testing in the pharmaceutical and medical device industries. Amebocyte Lysate is a crucial reagent used to detect bacterial endotoxins, ensuring the safety and sterility of injectable drugs, vaccines, and medical devices. As the global population ages and the demand for healthcare products increases, the need for reliable endotoxin testing methods has become more critical.
Moreover, advancements in biotechnology and the growing emphasis on food safety and environmental hygiene have expanded the applications of Amebocyte Lysate. It is increasingly used in food testing to detect bacterial contamination and in environmental monitoring to assess water quality and other environmental samples.
Figure Global Amebocyte Lysate Market Size (M USD) Outlook (2024-2033)
2 Amebocyte Lysate Market Driving Force
Table Market Driving Force
Item | Description |
Wide application of Amebocyte Lysate | When exposed to the toxin of the bacteria, the amoebocytes in the blood of the horseshoe crab release a coagulation protein, which causes the blood to coagulate rapidly, forming a barrier that prevents the bacteria from multiplying and prevents the invasion of other bacteria. Amebocyte Lysate can detect a contaminant called “endotoxin” (a type of bacteria), and can be widely used in the fields of medicine and hygiene, food hygiene, environmental hygiene, molecular biology, and microbiology. In the pharmaceutical industry, for example, even small amounts of endotoxin can be fatal in vaccines, injectable drugs, or other sterile medical items such as artificial knees and hip joints. Amebocyte Lysate can be used to test the safety of these products. In food hygiene, Amebocyte Lysate can be used to detect whether drinking water, milk, canned food and other foods are contaminated by bacteria. As people’s safety awareness continues to rise, the government’s supervision on medical hygiene and food hygiene will become more and more strict. The demand for Amebocyte Lysate in the market will continue to rise, thereby increasing the demand for Amebocyte Lysate. Overall, the wide application of Amebocyte Lysate is an important reason for the development of the industry. |
Development of the global medical industry | With the continuous expansion of the global population and the trend of aging population, the market size of the global medical industry continues to expand. At the same time, with the introduction of relevant policies by various governments to support the development of the medical industry, the global pharmaceutical industry continues to develop. For example, in China, “Made in China 2025” proposes to develop new products of chemical medicines, traditional Chinese medicines, and biotechnology medicines for major diseases. The Outline of the “Healthy China 2030” Plan vigorously develops new varieties of biological drugs, chemical drugs, and high-quality traditional Chinese medicines. The “Thirteenth Five-Year” National Strategic Emerging Industry Development Plan proposes to promote the innovation of chemical drugs and the development of high-end preparations. And Amebocyte Lysate is widely used in drug testing. After a long period of development and research, there are currently many kinds of Amebocyte Lysate, mainly including color matrix Amebocyte Lysate, gel method Amebocyte Lysate, dynamic turbidity method Amebocyte Lysate, specific Amebocyte Lysate, and freeze-dried powder injection Amebocyte Lysate, which can meet different need of the scene. Compared with other detection reagents, Amebocyte Lysate is easy to use, fast and sensitive, and has strong specificity. In terms of sensitivity, after testing, the sensitivity of Amebocyte Lysate is as high as 0.0001μg/ml, and only a small amount of endotoxin can be detected, which is 10 times higher than the traditional method. Therefore, with the development of the global medical industry, the market demand for Amebocyte Lysate will continue to rise. |
3 Global Amebocyte Lysate Market by Type in 2024
Amebocyte Lysate is a critical reagent derived from the blood of horseshoe crabs, used primarily for detecting bacterial endotoxins in various applications. In 2024, the market for Amebocyte Lysate was segmented into two main product types: Limulus Amebocyte Lysate (LAL) and Tachypleus Amebocyte Lysate (TAL).
Limulus Amebocyte Lysate (LAL)
LAL, derived from the Atlantic horseshoe crab (Limulus polyphemus), is the most widely used product type. It is a highly sensitive reagent that reacts with bacterial endotoxins to form a gel, indicating the presence of contamination. In 2024, LAL accounted for the largest market share, primarily due to its established use in the pharmaceutical industry for testing the sterility of injectable drugs, vaccines, and medical devices. The market size for LAL in 2024 was $642.26 million, representing approximately 88.68% of the total Amebocyte Lysate market. Its dominance can be attributed to its long-standing acceptance and reliability in endotoxin detection, as well as the extensive regulatory approvals and guidelines supporting its use.
Tachypleus Amebocyte Lysate (TAL)
TAL, sourced from the Asian horseshoe crab (Tachypleus tridentatus), is gaining traction due to its potential as an alternative to LAL. TAL offers similar sensitivity and specificity in detecting endotoxins but is less affected by certain interfering substances, making it advantageous in specific applications. In 2024, the market size for TAL was $75.96 million, comprising 11.32% of the total market. Although TAL has a smaller market share compared to LAL, it is experiencing a faster growth rate. This growth can be attributed to increasing awareness of its benefits, efforts to diversify the supply chain, and the need for alternative testing methods to mitigate the environmental impact of horseshoe crab harvesting.
The Amebocyte Lysate market in 2024 was dominated by Limulus Amebocyte Lysate, which accounted for the majority of the market share. However, Tachypleus Amebocyte Lysate showed significant promise, with a faster growth rate, indicating its potential to gain a larger market share in the future. As the industry continues to evolve, both product types will play crucial roles in meeting the diverse needs of endotoxin testing across various sectors, including pharmaceuticals, medical devices, and food safety.
Table Global Amebocyte Lysate Market Size and Share by Type in 2024
Type | Market Size (M USD) | Market Share |
|---|---|---|
Limulus Amebocyte Lysate | 642.26 | 88.68% |
Tachypleus Amebocyte Lysate | 75.96 | 11.32% |
Total | 718.22 | 100.00% |
4 Global Amebocyte Lysate Market by Application in 2024
Amebocyte Lysate is a versatile reagent used in various applications due to its ability to detect bacterial endotoxins. In 2024, the market was segmented into three primary applications: drug testing, clinical diagnosis, and other applications.
Drug Testing
Drug testing is the largest application area for Amebocyte Lysate. It involves the detection of endotoxins in pharmaceutical products, ensuring their safety and sterility. In 2024, the market size for drug testing was $483.69 million, representing approximately 67.32% of the total Amebocyte Lysate market. This dominance is attributed to the stringent regulatory requirements for endotoxin testing in the pharmaceutical industry. Manufacturers of injectable drugs, vaccines, and medical devices rely heavily on Amebocyte Lysate to comply with these regulations and ensure the safety of their products. The growing demand for new drugs and the expansion of the pharmaceutical industry globally have further fueled the market size for drug testing applications.
Clinical Diagnosis
Clinical diagnosis is another significant application of Amebocyte Lysate. It is used to detect endotoxins in clinical samples, aiding in the diagnosis of infections and other medical conditions. In 2024, the market size for clinical diagnosis was $228.14 million, accounting for 31.75% of the total market. The increasing prevalence of infectious diseases and the need for rapid and accurate diagnostic tools have driven the demand for Amebocyte Lysate in clinical settings. Additionally, advancements in medical technology and the growing awareness of the importance of early diagnosis have contributed to the market growth in this application.
The Amebocyte Lysate market in 2024 was primarily driven by its applications in drug testing and clinical diagnosis, with drug testing accounting for the largest market share. The growing demand for safe pharmaceutical products and accurate diagnostic tools has been a key factor in shaping the market dynamics. As the industry continues to evolve, Amebocyte Lysate will remain a crucial component in ensuring the safety and quality of various products and environments, supporting the growth and development of related sectors.
Table Global Amebocyte Lysate Market Size and Share by Application in 2024
Application | Market Size (M USD) | Market Share |
|---|---|---|
Drug Testing | 483.69 | 67.32% |
Clinical Diagnosis | 228.14 | 31.75% |
Other | 6.39 | 0.89% |
Total | 718.22 | 100.00% |
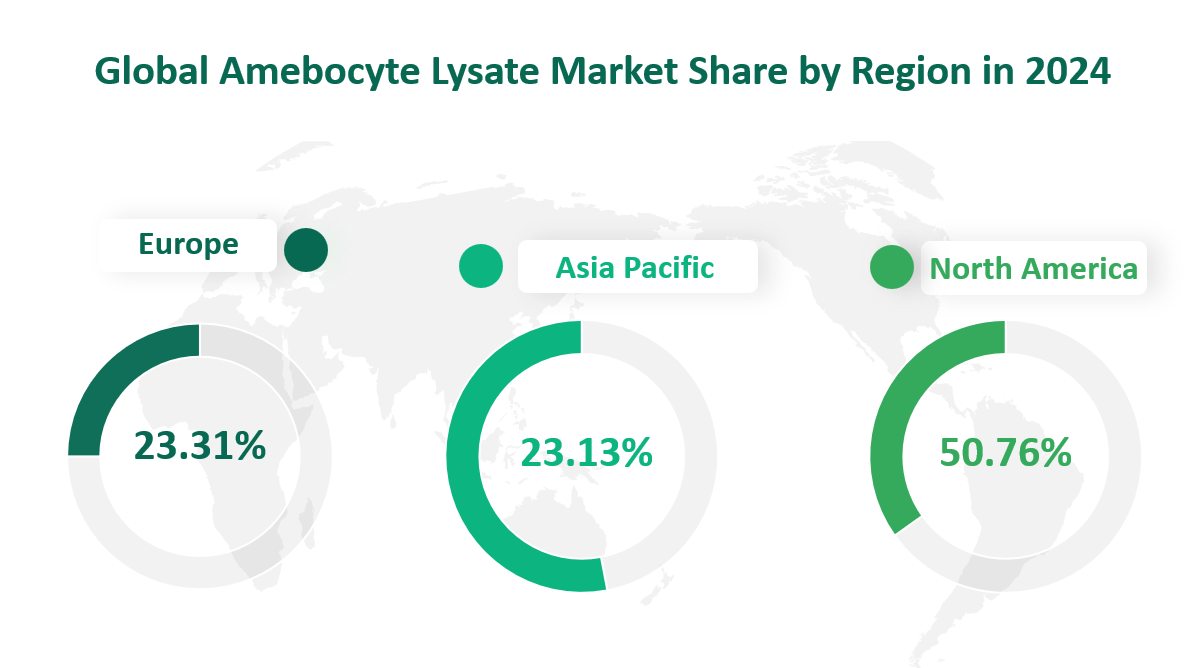
5 Global Amebocyte Lysate Market by Region in 2024
In 2024, the global Amebocyte Lysate market exhibited significant growth and expansion, with different regions contributing to its overall value. The market was segmented into several key regions: North America, Europe, Asia-Pacific, South America, and the Middle East & Africa.
North America
North America emerged as the largest regional market by sales in 2024, generating 64051 K Units. This region accounted for approximately 50.76% of the global market share. The dominance of North America can be attributed to several factors. Firstly, the presence of major pharmaceutical companies and advanced healthcare infrastructure in the United States and Canada drives the demand for Amebocyte Lysate. These companies rely heavily on endotoxin testing to ensure the safety and sterility of their products, including injectable drugs, vaccines, and medical devices. Additionally, the region’s strong focus on research and development in biotechnology and pharmaceuticals further supports the market growth. The stringent regulatory requirements for product safety also necessitate the use of reliable testing methods, such as Amebocyte Lysate, contributing to its high market share.
Europe
Europe was the second-largest regional market in 2024, with a sales of 29410 K Units, representing 23.31% of the global market share. The European market is characterized by a robust healthcare sector and a growing emphasis on quality control in pharmaceutical and medical device manufacturing. Countries like Germany, the UK, and France have established pharmaceutical industries that require extensive endotoxin testing to comply with regulatory standards. The European Medicines Agency (EMA) and other regulatory bodies enforce strict guidelines for product safety, driving the demand for Amebocyte Lysate. Furthermore, the region’s investment in research and development, particularly in biotechnology and healthcare, supports the market’s growth. Europe’s focus on innovation and sustainability also encourages the development and adoption of advanced testing methods, including Amebocyte Lysate.
Asia-Pacific
The Asia-Pacific region experienced significant growth in 2024, with a market sales of 29184 K Units, comprising 23.13% of the global market share. This region is rapidly emerging as a key player in the Amebocyte Lysate market due to its expanding pharmaceutical and biotechnology industries. Countries like China, Japan, and South Korea are experiencing substantial growth in the production of pharmaceuticals and medical devices, driving the demand for endotoxin testing. The increasing awareness of product safety and quality standards in these countries has led to the adoption of Amebocyte Lysate as a reliable testing method. Additionally, the region’s growing population and rising healthcare expenditures contribute to the market’s expansion. The Asia-Pacific region is also benefiting from government initiatives and investments in healthcare infrastructure, further supporting the growth of the Amebocyte Lysate market.
South America
South America contributed 1932 K Units to the global Amebocyte Lysate market in 2024, accounting for 1.53% of the market share. While this region has a smaller market share compared to others, it is experiencing steady growth. The pharmaceutical industry in countries like Brazil and Argentina is expanding, driving the demand for endotoxin testing. The region’s focus on improving healthcare standards and ensuring product safety is leading to increased adoption of Amebocyte Lysate. Additionally, the growing biotechnology sector and investments in research and development are contributing to the market’s growth potential in South America.
Middle East & Africa
The Middle East & Africa region had a market sales of 1619 K Units in 2024, representing 1.28% of the global market share. This region is gradually gaining prominence in the Amebocyte Lysate market due to its developing healthcare sector and increasing emphasis on pharmaceutical manufacturing. Countries like Saudi Arabia and the United Arab Emirates are investing in healthcare infrastructure and biotechnology, driving the demand for endotoxin testing. The need for safe and effective pharmaceutical products in the region is leading to the adoption of Amebocyte Lysate as a reliable testing method. Furthermore, the region’s growing population and rising healthcare expenditures are contributing to the market’s expansion.
In conclusion, the Amebocyte Lysate market in 2024 was characterized by significant growth across major regions. North America emerged as the largest regional market by revenue, while the Asia-Pacific region demonstrated the fastest growth rate. Each region contributed to the overall market dynamics, driven by factors such as the pharmaceutical industry’s expansion, increasing healthcare standards, and investments in research and development. As the global healthcare sector continues to evolve, the demand for Amebocyte Lysate is expected to persist, with regions like Asia-Pacific playing an increasingly prominent role in the market’s growth.
Table Global Amebocyte Lysate Market Sales, Region Wise in 2024
Region | Market Sales (K Units) | Market Share |
|---|---|---|
North America | 64051 | 50.76% |
Europe | 29410 | 23.31% |
Asia-Pacific | 29184 | 23.13% |
South America | 1932 | 1.53% |
Middle East & Africa | 1619 | 1.28% |
Total | 126196 | 100.00% |
Figure Global Amebocyte Lysate Sales Market Share by Region in 2024
6 Global Amebocyte Lysate Market Top 3 Players
Company Introduction and Business Overview: Lonza is a leading global provider of integrated healthcare solutions, with a strong focus on enabling treatments that prevent diseases and support healthier lifestyles. Founded in 1897, Lonza has a global presence with manufacturing facilities primarily located in Europe and the United States. The company offers a wide range of products and services, including biopharmaceuticals, pharmaceutical ingredients, and healthcare ingredients. Lonza’s commitment to scientific innovation and manufacturing excellence has positioned it as a key player in the Amebocyte Lysate market.
Products Offered: Lonza’s Amebocyte Lysate products include Limulus Amebocyte Lysate (LAL) PYROGENT™ and PYROGENT™Plus. These products are designed for in vitro endotoxin testing of human and animal parenteral drugs, biological products, and medical devices. The LAL test is a qualitative assay for Gram-negative bacterial endotoxin, providing reliable and accurate results. Lonza’s LAL products are known for their high sensitivity, specificity, and ease of use, making them suitable for various testing applications.
Revenue in 2022: In 2022, Lonza’s revenue from Amebocyte Lysate products was $280.78 million. This figure reflects the company’s strong market position and the continued demand for its innovative and reliable testing solutions. Lonza’s revenue growth can be attributed to its extensive product portfolio, global customer base, and ongoing investments in research and development.
Company Introduction and Business Overview: Charles River Laboratories is a renowned American pharmaceutical company specializing in preclinical and clinical laboratory services, gene therapy, and cell therapy services. Founded in 1947, the company operates primarily in the United States and serves a diverse range of clients in the pharmaceutical, medical device, and biotechnology industries. Charles River Laboratories also provides various biomedical products and outsourcing services, supporting research and development efforts in drug discovery, safety, and efficacy.
Products Offered: The company offers a comprehensive range of Amebocyte Lysate products, including Endosafe® Cartridge Technology, Kinetic Chromogenic LAL, and Kinetic Turbidimetric LAL. The Endosafe® cartridge technology is an advanced solution that enhances the sensitivity and speed of LAL testing, reducing the need for raw materials and preparation time. Kinetic Chromogenic LAL is a fully quantitative and stable reagent, delivering high sensitivity and resistance to interference. Kinetic Turbidimetric LAL allows for both kinetic and gel-clot analysis with accelerated reaction times, providing a versatile testing option.
Revenue in 2022: In 2022, Charles River Laboratories generated a revenue of $177.53 million from its Amebocyte Lysate products. This revenue figure highlights the company’s strong market presence and the effectiveness of its innovative product offerings. Charles River Laboratories’ revenue growth can be attributed to its diverse product portfolio, extensive customer base, and continuous focus on improving and refining its testing technologies.
Company Introduction and Business Overview: Associates of Cape Cod, Inc. (ACC) is a leading provider of endotoxin and (1→3)-ß-D-glucan testing products and services. Founded in 1974, ACC is headquartered in the United States and has been at the forefront of Limulus test method development. The company is internationally recognized for its expertise in endotoxin testing and has received FDA clearance to manufacture Limulus reagents. ACC’s commitment to innovation and quality has established it as a key player in the Amebocyte Lysate market.
Products Offered: ACC offers a variety of Amebocyte Lysate products, including Chromo-LAL, a chromogenic lysate optimized for the kinetic chromogenic LAL test method. Chromo-LAL is a stable and robust lysate suitable for quantitative testing of a wide range of samples. The product is designed to provide high sensitivity and specificity, ensuring accurate endotoxin detection. ACC’s product offerings also include other endotoxin testing reagents and related accessories, catering to the diverse needs of customers in the pharmaceutical, biotechnology, and medical device industries.
Revenue in 2022: In 2024, Associates of Cape Cod achieved a revenue of $44.91 million from its Amebocyte Lysate products. This revenue figure demonstrates the company’s strong market position and the effectiveness of its innovative testing solutions. ACC’s revenue growth can be attributed to its extensive product range, established reputation in the industry, and ongoing commitment to providing high-quality products and services.
Table Global Amebocyte Lysate Revenue of Top3 Players in 2022
Company | Revenue (M USD) |
LONZA | 280.78 |
Charles River Laboratories | 177.53 |
Associates of Cape Cod | 44.91 |

